无机材料学报 ›› 2021, Vol. 36 ›› Issue (12): 1330-1336.DOI: 10.15541/jim20210063 CSTR: 32189.14.10.15541/jim20210063
收稿日期:2021-02-01
修回日期:2021-03-30
出版日期:2021-12-20
网络出版日期:2021-04-05
通讯作者:
张 恒, 博士, 副教授. E-mail: zhangheng@qfnu.edu.cn
作者简介:王婷婷(1979-), 女, 硕士. E-mail: wangting@qfnu.edu.cn
WANG Tingting( ), SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng(
), SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng( )
)
Received:2021-02-01
Revised:2021-03-30
Published:2021-12-20
Online:2021-04-05
Contact:
ZHANG Heng, PhD, associate professor. E-mail: zhangheng@qfnu.edu.cn
About author:WANG Tingting (1979-), female, Master. E-mail: wangting@qfnu.edu.cn
Supported by:摘要:
层状硅酸镍因其独特的结构, 在电化学和催化等领域展现出良好的应用前景, 其合成与性能研究近年来受到广泛关注。本研究以氯化镍和正硅酸乙酯为原料, 采用水热法合成了硅酸镍微球, 并详细探究了镍硅比和碱源对产物组成、形貌及孔结构的影响。在优化条件下, 产物呈现由纳米片组装的、平均直径约为2.5 μm的微球形貌, 比表面积为119.6 m2·g-1, 孔容为0.673 cm3·g-1。Zeta电位分析表明, 该微球在pH=3~10范围内保持表面电负性。将硅酸镍微球用于处理碱性品红溶液, 吸附过程符合准二级动力学模型。在初始浓度为50 mg·L-1的条件下, 吸附容量可达120.7 mg·g-1, 脱除率达96.6%, 远优于改性粘土及近年来报道的多种材料。吸附量与平衡浓度的数据表明, 碱性品红在硅酸镍微球上的吸附符合Freundlich吸附模型, 1/n=0.1678, 表明该吸附为多层非均相吸附且吸附作用力强。
中图分类号:
王婷婷, 史书梅, 柳晨媛, 朱万诚, 张恒. 多级多孔硅酸镍微球的合成及其对碱性品红的高效吸附[J]. 无机材料学报, 2021, 36(12): 1330-1336.
WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin[J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336.
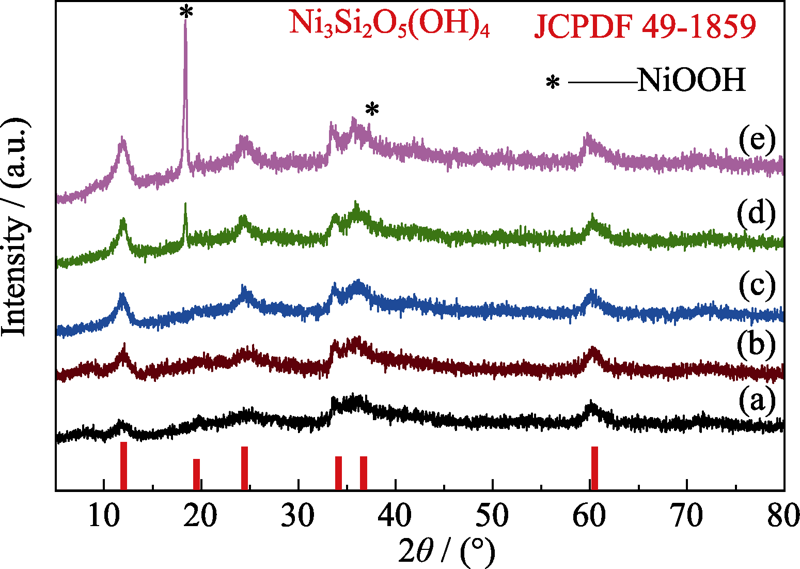
Fig. 1 Effect of Ni/Si molar ratio on the phase composition of the products hydrothermally synthesized at 210 ℃ for 12 h with different Ni/Si molar ratios (a) 0.5 : 1; (b) 0.75 : 1; (c) 1 : 1; (d) 1.25 : 1; (e) 1.5 : 1
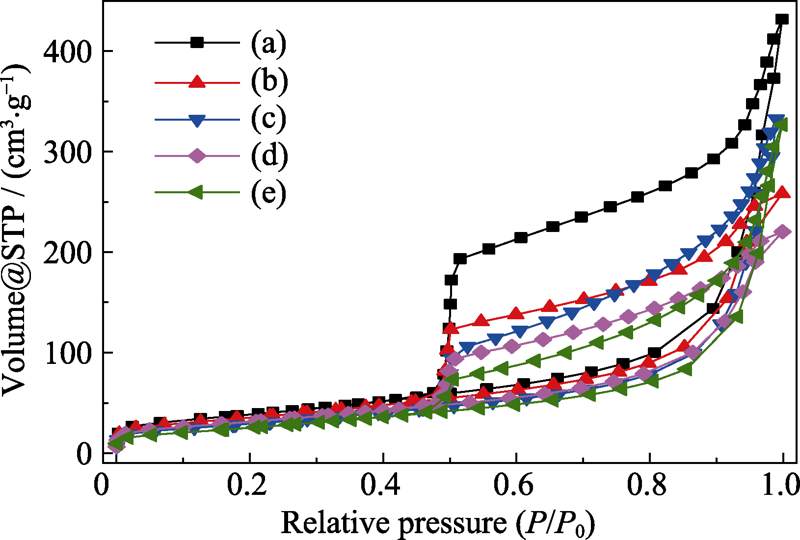
Fig. 3 N2 adsorption-desorption isotherms of the products hydrothermally synthesized at 210 ℃ for 12 h with different Ni/Si molar ratios (a) 0.5 : 1; (b) 0.75 : 1; (c) 1 : 1; (d) 1.25 : 1; (e) 1.5 : 1
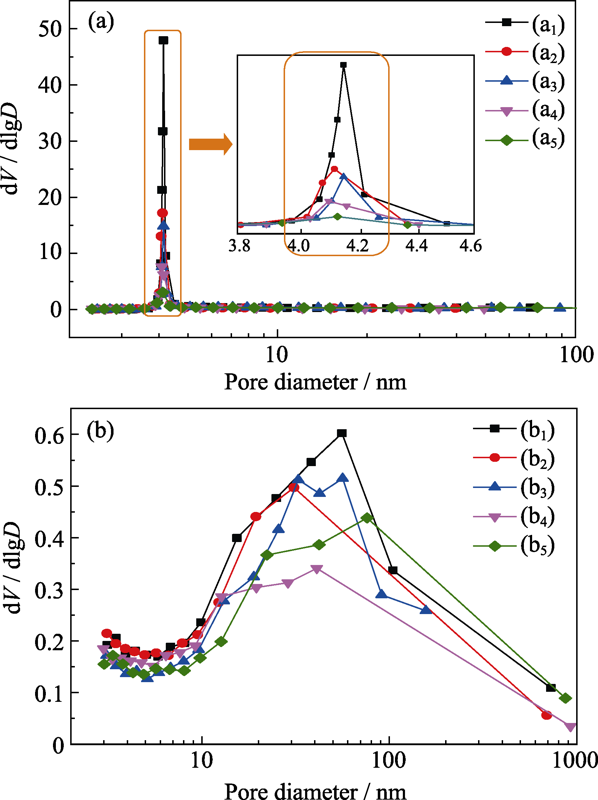
Fig. 4 Pore size distribution derived from desorption (a) and adsorption (b) branch of the isotherm of the products hydrothermally synthesized with different Ni/Si molar ratios (a1, b1) 0.5 : 1; (a2, b2) 0.75 : 1; (a3, b3) 1 : 1; (a4, b4) 1.25 : 1; (a5, b5) 1.5 : 1
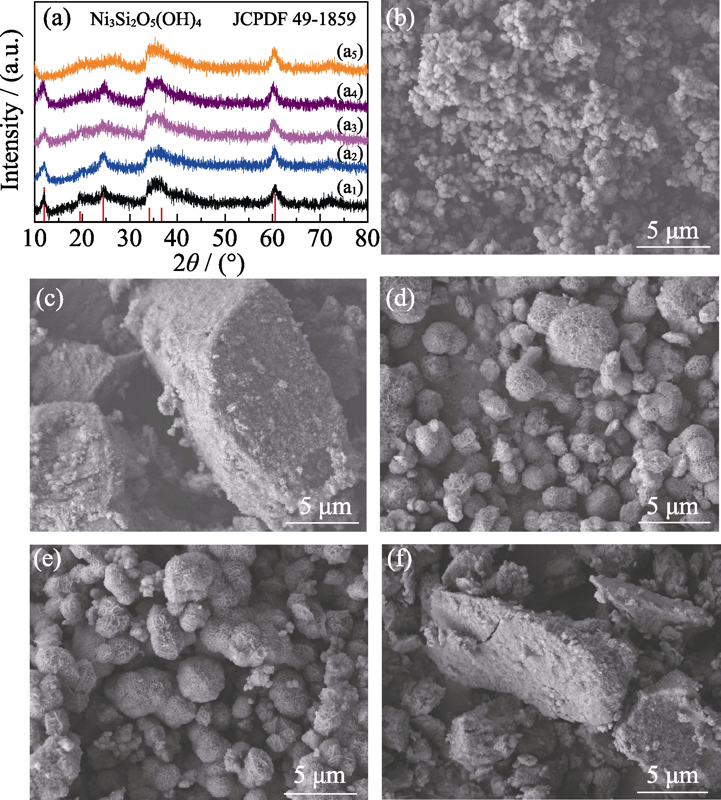
Fig. 5 Effect of alkali source on composition and morphology of the products (a1, b) Ammonia, 26.6 mmol; (a2, c) Sodium hydroxide, 26.6 mmol; (a3, d) Urea, 3.33 mmol; (a4, e) Urea, 6.66 mmol; (a5, f) Urea, 20.0 mmol
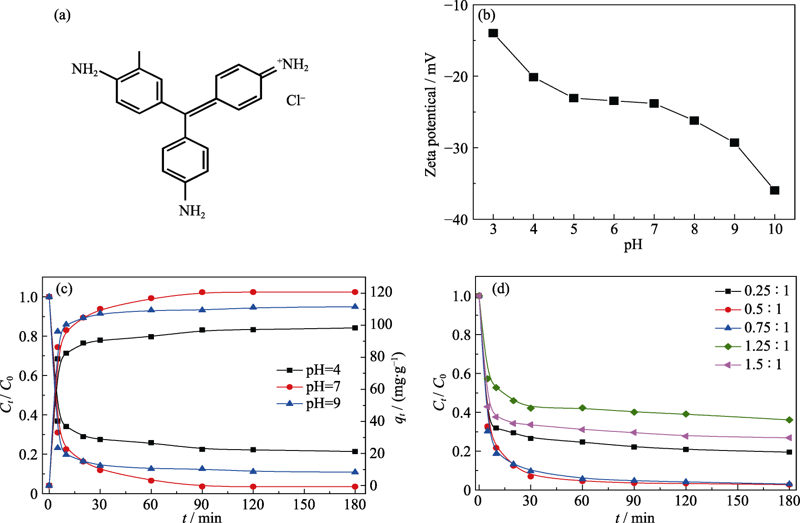
Fig. 7 Molecular structure of BF (a), Zeta potential of Ni3Si2O5(OH)4 microspheres (Ni/Si molar ratio of 1 : 1) (b), variation of the adsorption rate and capacity with adsorption time at different pH (Ni/Si molar ratio of 1 : 1) (c), and effect of Ni/Si molar ratio on the adsorption performance (d)
| Ni/Si molar ratio | SBET /(m2·g-1) | Pore volume /(cm3·g-1) | Average pore diameter/nm |
|---|---|---|---|
| 0.5 : 1 | 139.4 | 0.884 | 6.00 |
| 0.75 : 1 | 128.2 | 0.511 | 5.58 |
| 1 : 1 | 119.6 | 0.673 | 5.90 |
| 1.25 : 1 | 101.1 | 0.426 | 5.86 |
| 1.5 : 1 | 95.5 | 0.564 | 8.68 |
Table S1 Textural properties of the products
| Ni/Si molar ratio | SBET /(m2·g-1) | Pore volume /(cm3·g-1) | Average pore diameter/nm |
|---|---|---|---|
| 0.5 : 1 | 139.4 | 0.884 | 6.00 |
| 0.75 : 1 | 128.2 | 0.511 | 5.58 |
| 1 : 1 | 119.6 | 0.673 | 5.90 |
| 1.25 : 1 | 101.1 | 0.426 | 5.86 |
| 1.5 : 1 | 95.5 | 0.564 | 8.68 |
| qe,exp /(mg·g-1) | Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|
| qe,calc1/(mg·g-1) | k1/min-1 | R2 | qe,calc2/(mg·g-1) | k2/(mg·g-1·min-1) | R2 | |
| 120.7 | 55.2 | 0.0483 | 0.8526 | 118.5 | 0.0051 | 0.9979 |
Table S2 Adsorption kinetic model parameters for the adsorption of BF on the Ni3Si2O5(OH)4 microspheres
| qe,exp /(mg·g-1) | Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|
| qe,calc1/(mg·g-1) | k1/min-1 | R2 | qe,calc2/(mg·g-1) | k2/(mg·g-1·min-1) | R2 | |
| 120.7 | 55.2 | 0.0483 | 0.8526 | 118.5 | 0.0051 | 0.9979 |
| Adsorbents | Initial concentration of BF solution/(mg·L-1) | Adsorption equilibrium time/min | Maximum adsorption capacity /(mg·g-1) | Ref. |
|---|---|---|---|---|
| Alkali-activated diatomite | 15 | 30 | 4.85 | [1] |
| (Acrylamide-co-sodium methacrylate )-graft-chitosan gel | 125 | 180 | 6.1 | [2] |
| β-cyclodextrin-carboxymethyl cellulose-graphene oxide composite | 100 | 150 | 6.5 | [3] |
| Hydroxy-aluminum pillared bentonite | 100 | 10-15 | 6.6 | [4] |
| Iron-manganese oxide coated kaolinite | 40 | 50 | 8.16 | [5] |
| Copper vinylphosphonate | 30 | 150 | 19.29 | [6] |
| Fe-ZSM-5 | 20 | 240 | 25.8 | [7] |
| β-cyclodextrin-styrene-based polymer | 50 | 180 | 29.6 | [8] |
| CoFe2O4-HA-ECH | 33.8 | 30 | 31.3 | [9] |
| Magnetic chitosan/graphene oxide | 50 | 70 | 32.8 | [10] |
| Activated carbon/ferrospinel composite | 100 | 60 | 49.9 | [11] |
| Al-MCM-41 | 60 | 240 | 54 | [12] |
| Ba(B2Si2O8) microspheres | 30 | 240 | 58.0 | [13] |
| NiFe2O4/polythiophene nanocomposite | 50 | 30 | 76 | [14] |
| Ni3Si2O5(OH)4 | 50 | 180 | 120.7 | This work |
Table S3 Comparison of the adsorption capacities for BF on various adsorbents
| Adsorbents | Initial concentration of BF solution/(mg·L-1) | Adsorption equilibrium time/min | Maximum adsorption capacity /(mg·g-1) | Ref. |
|---|---|---|---|---|
| Alkali-activated diatomite | 15 | 30 | 4.85 | [1] |
| (Acrylamide-co-sodium methacrylate )-graft-chitosan gel | 125 | 180 | 6.1 | [2] |
| β-cyclodextrin-carboxymethyl cellulose-graphene oxide composite | 100 | 150 | 6.5 | [3] |
| Hydroxy-aluminum pillared bentonite | 100 | 10-15 | 6.6 | [4] |
| Iron-manganese oxide coated kaolinite | 40 | 50 | 8.16 | [5] |
| Copper vinylphosphonate | 30 | 150 | 19.29 | [6] |
| Fe-ZSM-5 | 20 | 240 | 25.8 | [7] |
| β-cyclodextrin-styrene-based polymer | 50 | 180 | 29.6 | [8] |
| CoFe2O4-HA-ECH | 33.8 | 30 | 31.3 | [9] |
| Magnetic chitosan/graphene oxide | 50 | 70 | 32.8 | [10] |
| Activated carbon/ferrospinel composite | 100 | 60 | 49.9 | [11] |
| Al-MCM-41 | 60 | 240 | 54 | [12] |
| Ba(B2Si2O8) microspheres | 30 | 240 | 58.0 | [13] |
| NiFe2O4/polythiophene nanocomposite | 50 | 30 | 76 | [14] |
| Ni3Si2O5(OH)4 | 50 | 180 | 120.7 | This work |
| Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|
| qm/(mg·g-1) | b/(L·mg-1) | R2 | kf | 1/n | R2 |
| 176.7 | 4.7474 | 0.7920 | 104.9 | 0.1678 | 0.9919 |
Table S4 Corresponding fitting parameters originated from the non-linear regression by using Langmuir and Freundlich isotherm models
| Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|
| qm/(mg·g-1) | b/(L·mg-1) | R2 | kf | 1/n | R2 |
| 176.7 | 4.7474 | 0.7920 | 104.9 | 0.1678 | 0.9919 |
| [1] |
RICHARD-PLOUET M, VILMINOT S, GUILLOT M. Synthetic transition metal phyllosilicates and organic-inorganic related phases. New Journal of Chemistry, 2004, 28(9): 1073-1082.
DOI URL |
| [2] | MUNIRASU S, AGGARWAL R, BASKARAN D. Highly efficient recyclable hydrated-clay supported catalytic system for atom transfer radical polymerization. Chemical Communications, 2009(30): 4518-4520. |
| [3] |
SOETAREDJO F E, AYUCITRA A, ISMADJI S, et al. KOH/ bentonite catalysts for transesterification of palm oil to biodiesel. Applied Clay Science, 2011, 53(2): 341-346.
DOI URL |
| [4] |
BIAN Z, KAWI S. Preparation, characterization and catalytic application of phyllosilicate: a review. Catalysis Today, 2020, 339: 3-23.
DOI URL |
| [5] |
HERNEY-RAMIREZ J, VICENTE MA, MADEIRA LM. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: a review. Applied Catalysis B: Environmental, 2010, 98(1): 10-26.
DOI URL |
| [6] |
KUMAR DUTTA D, JYOTI BORAH B, POLLOV SARMAH P. Recent advances in metal nanoparticles stabilization into nanopores of montmorillonite and their catalytic applications for fine chemicals synthesis. Catalysis Reviews, 2015, 57(3): 257-305.
DOI URL |
| [7] |
JIANG B, LI L, BIAN Z, et al. Hydrogen generation from chemical looping reforming of glycerol by Ce-doped nickel phyllosilicate nanotube oxygen carriers. Fuel, 2018, 222: 185-192.
DOI URL |
| [8] |
GHIAT I, BOUDJEMAA A, SAADI A, et al. Efficient hydrogen generation over a novel Ni phyllosilicate photocatalyst. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 382: 111952.
DOI URL |
| [9] |
LU Y, GUO D, ZHAO Y, et al. Confined high dispersion of Ni nanoparticles derived from nickel phyllosilicate structure in silicalite-2 shell for dry reforming of methane with enhanced performance. Microporous and Mesoporous Materials, 2021, 313: 110842.
DOI URL |
| [10] |
KIM B, KIM JS, KIM H, et al. Amorphous multinary phyllosilicate catalysts for electrochemical water oxidation. Journal of Materials Chemistry A, 2019, 7(31): 18380-18387.
DOI URL |
| [11] |
DI W, CHENG J, TIAN S, et al. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogennation to ethanol. Applied Catalysis A: General, 2016, 510: 244-259.
DOI URL |
| [12] |
BIAN Z, ZHONG W, YU Y, et al. Cu/SiO2 derived from copper phyllosilicate for low-temperature water-gas shift reaction: role of Cu+ sites. International Journal of Hydrogen Energy, 2020, 45(51): 27078-27088.
DOI URL |
| [13] | LEE Y C, KIM E J, YANG J W, et al. Removal of malachite green by adsorption and precipitation using aminopropyl functionalized magnesium phyllosilicate. Journal of Hazardous Materials, 2011, 192(1): 62-70. |
| [14] |
SIVAIAH M V, PETIT S, BEAUFORT M F, et al. Nickel based catalysts derived from hydrothermally synthesized 1 : 1 and 2 : 1 phyllosilicates as precursors for carbon dioxide reforming of methane. Microporous and Mesoporous Materials, 2011, 140(1/2/3): 69-80.
DOI URL |
| [15] |
MCDONALD A, SCOTT B, VILLEMURE G. Hydrothermal preparation of nanotubular particles of a 1 : 1 nickel phyllosilicate. Microporous and Mesoporous Materials, 2009, 120(3): 263-266.
DOI URL |
| [16] |
YANG Y, LIANG Q, LI J, et al. Ni3Si2O5(OH)4 multi-walled nanotubes with tunable magnetic properties and their application as anode materials for lithium batteries. Nano Research, 2011, 4(9): 882-890.
DOI URL |
| [17] |
WHITE R D, BAVYKIN D V, WALSH F C. Morphological control of synthetic Ni3Si2O5(OH)4 nanotubes in an alkaline hydrothermal environment. Journal of Materials Chemistry A, 2013, 1(3): 548-556.
DOI URL |
| [18] | GUO Z, DU F, LI G, et al. Controlled synthesis of mesoporous SiO2/Ni3Si2O5(OH)4 core-shell microspheres with tunable chamber structures via a self-template method. Chemical Communications, 2008(25): 2911-2913. |
| [19] |
CHEN D, GUO Z, SUN T, et al. Controlled synthesis and catalytic properties of mesoporous nickel-silica core-shell microspheres with tunable chamber structures. Materials Research Bulletin, 2012, 47(9): 2344-2348.
DOI URL |
| [20] |
WANG T, LIU C, MA X, et al. Synthesis of Ni3Si4O10(OH)2 porous microspheres as support of Pd catalyst for hydrogenation reaction. Nanomaterials, 2019, 9(7): 998.
DOI URL |
| [21] |
GROEN J C, PEFFER L A A, PÉREZ-RAMÍREZ J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous and Mesoporous Materials, 2003, 60(1/2/3): 1-17.
DOI URL |
| [22] |
DONG F, XIONG T, WANG R, et al. Growth mechanism and photocatalytic activity of self-organized N-doped (BiO)2CO3 hierarchical nanosheet microspheres from bismuth citrate and urea. Dalton Transactions, 2014, 43(18): 6631-6642.
DOI URL |
| [23] |
LI J, XU L, SUN P, et al. Novel application of red mud: facile hydrothermal-thermal conversion synthesis of hierarchical porous AlOOH and Al2O3 microspheres as adsorbents for dye removal. Chemical Engineering Journal, 2017, 321: 622-634.
DOI URL |
| [24] |
MCKAY G, BLAIR H S, GARDNER J R. Adsorption of dyes on chitin. I. Equilibrium studies. Journal of Applied Polymer Science, 1982, 27(8): 3043-3057.
DOI URL |
| [1] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [2] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [3] | 洪培萍, 梁龙, 吴炼, 马颖康, 庞浩. ZIF-67结构调控及其对盐酸金霉素的吸附性能研究[J]. 无机材料学报, 2025, 40(4): 388-396. |
| [4] | 冯关正, 杨健, 周渡, 陈啟明, 许文涛, 周有福. 水热-碳热合成AlN纳米粉体的机理[J]. 无机材料学报, 2025, 40(1): 104-110. |
| [5] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [6] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [7] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [8] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [9] | 郭春霞, 陈伟东, 闫淑芳, 赵学平, 杨傲, 马文. 埃洛石纳米管负载锆氧化物吸附水中砷的研究[J]. 无机材料学报, 2023, 38(5): 529-536. |
| [10] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [11] | 于业帆, 徐玲, 倪忠斌, 施冬健, 陈明清. 普鲁士蓝/生物炭材料的制备及其氨氮吸附机理[J]. 无机材料学报, 2023, 38(2): 205-212. |
| [12] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [13] | 汤亚, 孙盛睿, 樊佳, 杨庆峰, 董满江, 寇佳慧, 刘阳桥. 粉煤灰衍生水合硅酸钙PEI改性及吸附去除Cu(II)与催化降解有机污染物[J]. 无机材料学报, 2023, 38(11): 1281-1291. |
| [14] | 戴洁燕, 冯爱虎, 米乐, 于洋, 崔苑苑, 于云. NaY沸石分子吸附涂层对典型空间污染物的吸附机制研究[J]. 无机材料学报, 2023, 38(10): 1237-1244. |
| [15] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||