无机材料学报 ›› 2021, Vol. 36 ›› Issue (7): 753-760.DOI: 10.15541/jim20200657 CSTR: 32189.14.10.15541/jim20200657
许宏一1( ), 翟东2, 曹琬婷1, 陈振华3, 钱文昊1(
), 翟东2, 曹琬婷1, 陈振华3, 钱文昊1( ), 陈蕾2(
), 陈蕾2( )
)
收稿日期:2020-11-18
修回日期:2020-12-22
出版日期:2021-07-20
网络出版日期:2020-12-30
通讯作者:
钱文昊, 主任医师. E-mail:pingyanlaoto@163.com;陈蕾, 高级工程师. E-mail:chenlei@mail.sic.ac.cn
作者简介:许宏一(1988-), 男, 主管技师. E-mail:13585840803@139.com
基金资助:
XU Hongyi1( ), ZHAI Dong2, CAO Wanting1, CHEN Zhenhua3, QIAN Wenhao1(
), ZHAI Dong2, CAO Wanting1, CHEN Zhenhua3, QIAN Wenhao1( ), CHEN Lei2(
), CHEN Lei2( )
)
Received:2020-11-18
Revised:2020-12-22
Published:2021-07-20
Online:2020-12-30
Contact:
QIAN Wenhao, chief physician. E-mail:pingyanlaoto@163.com;CHEN Lei, senior engineer. E-mail:chenlei@mail.sic.ac.cn
About author:XU Hongyi(1988-), male, technologist-in-charge. E-mail:13585840803@139.com
Supported by:摘要:
如何有效治疗牙周炎并实现受损牙周骨组织再生, 一直是牙周疾病治疗中具有挑战性的问题, 而矿化是牙周正常发育和功能中关键因素之一。本研究旨在探讨硅酸钙锂(Li2Ca2Si2O7)生物陶瓷对人牙周膜成纤维细胞增殖、矿化的影响及用于牙周骨组织再生的可能性。采用溶胶-凝胶法制备合成了Li2Ca2Si2O7陶瓷粉体。通过体外模拟体液浸泡, 发现Li2Ca2Si2O7粉体具有良好的羟基磷灰石矿化能力。生物学结果表明: Li2Ca2Si2O7粉体的浸提液在3.125~25 mg/mL浓度范围内能显著促进HPLFs的增殖, 低浓度(6.25 mg/mL)时可显著诱导HPLFs细胞体外矿化(p<0.05)。Li2Ca2Si2O7粉体具有促进人牙周膜成纤维细胞增殖和矿化能力, 有望作为牙周骨组织再生修复的生物活性材料。
中图分类号:
许宏一, 翟东, 曹琬婷, 陈振华, 钱文昊, 陈蕾. Li2Ca2Si2O7生物陶瓷的矿化活性研究[J]. 无机材料学报, 2021, 36(7): 753-760.
XU Hongyi, ZHAI Dong, CAO Wanting, CHEN Zhenhua, QIAN Wenhao, CHEN Lei. Mineralization Activity of Li2Ca2Si2O7 Bioceramics[J]. Journal of Inorganic Materials, 2021, 36(7): 753-760.
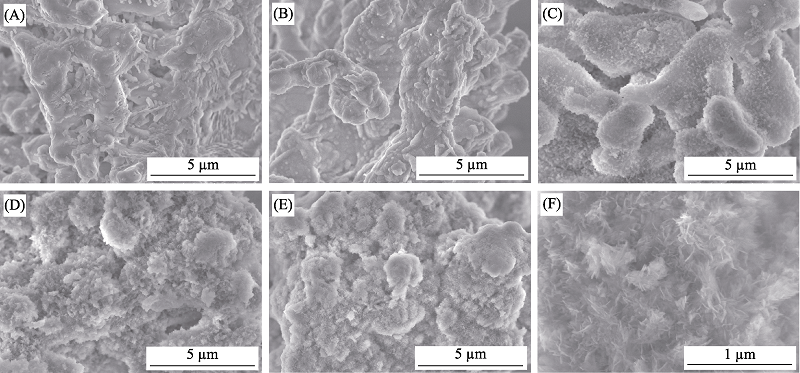
图3 Li2Ca2Si2O7粉体浸泡SBF不同时间后的SEM照片
Fig. 3 SEM images of Li2Ca2Si2O7 powders soaked in SBF for different periods (A) 0 d; (B) 1 d; (C) 3 d; (D) 7 d; (E) 14 d; (F) 14 d (enlarge image)
| Ionic concentration/(mg·L-1) | Powder extract concentrations /(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | 3.125 | 6.25 | 12.5 | 25 | 50 | 100 | 200 | |
| Li | 0 | 13.45 | 27.31 | 53.42 | 114.31 | 225.79 | 440.40 | 864.14 |
| Ca | 69.46 | 68.61 | 68.91 | 65.46 | 60.36 | 56.82 | 46.04 | 23.16 |
| Si | 1.19 | 13.62 | 26.16 | 51.57 | 102.70 | 205.01 | 416.02 | 840.13 |
| P | 28.37 | 27.48 | 26.44 | 25.28 | 22.19 | 19.66 | 13.94 | 0.71 |
表1 Li2Ca2Si2O7粉体浸提液的离子浓度
Table 1 Ion concentrations of Li2Ca2Si2O7 powders extracts
| Ionic concentration/(mg·L-1) | Powder extract concentrations /(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | 3.125 | 6.25 | 12.5 | 25 | 50 | 100 | 200 | |
| Li | 0 | 13.45 | 27.31 | 53.42 | 114.31 | 225.79 | 440.40 | 864.14 |
| Ca | 69.46 | 68.61 | 68.91 | 65.46 | 60.36 | 56.82 | 46.04 | 23.16 |
| Si | 1.19 | 13.62 | 26.16 | 51.57 | 102.70 | 205.01 | 416.02 | 840.13 |
| P | 28.37 | 27.48 | 26.44 | 25.28 | 22.19 | 19.66 | 13.94 | 0.71 |
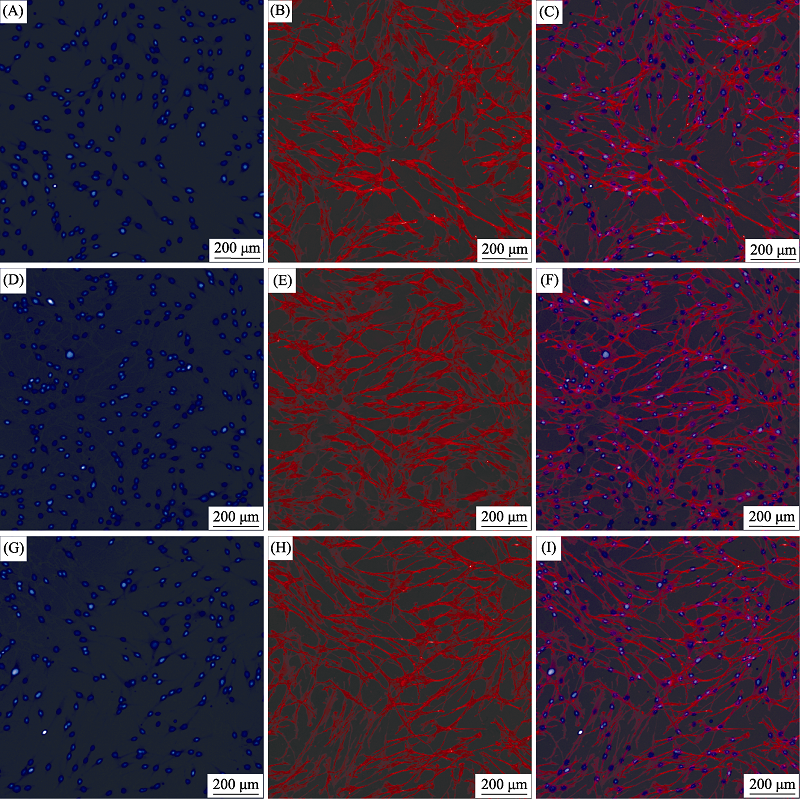
图6 Li2Ca2Si2O7粉体浸提液诱导HPLFs细胞骨架染色照片
Fig. 6 Cytoskeleton staining of HPLFs induced by Li2Ca2Si2O7 powders extract (A-C) Blank control; (D-F) 6.25 mg/mL; (G-I) 50 mg/mL; Blue: Nucleus; Red: Cytoskeleton
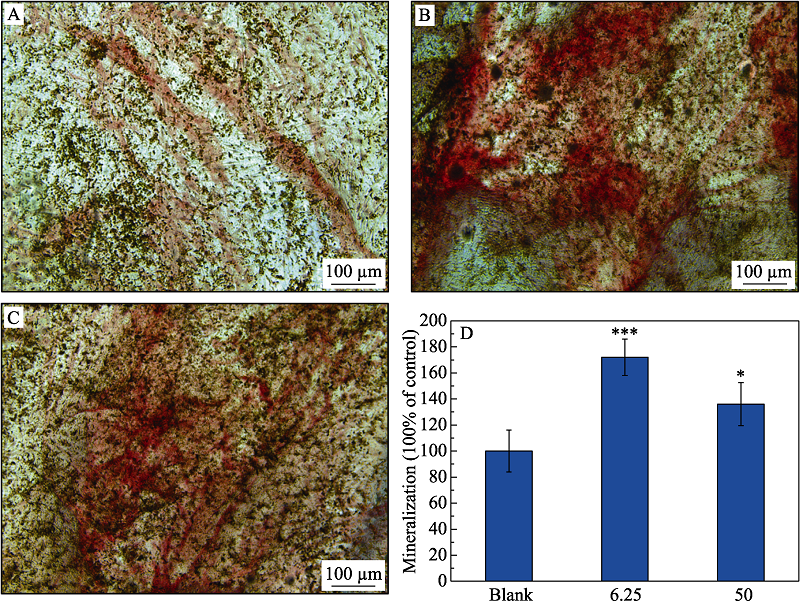
图7 Li2Ca2Si2O7粉体浸提液诱导HPLFs细胞茜素红染色图
Fig. 7 Alizarin red staining of HPLFs induced by Li2Ca2Si2O7 powders extract (A) Blank control; (B) 6.25 mg/mL; (C) 50 mg/mL; (D) Mineralization. *: p<0.05; ***: p<0.001
| [1] |
JEPSEN S, CATON J G, ALBANDAR J M, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology, 2018,45(Suppl 20):S219-S229.
DOI URL |
| [2] |
CHEN F M, JIN Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Engineering Part B-Reviews, 2010,16(2):219-255.
DOI URL |
| [3] |
NAKASHIMA M, REDDI A H. The application of bone morphogenetic proteins to dental tissue engineering. Nature Biotechnology, 2003,21:1025-1032.
DOI URL |
| [4] |
CHEN F M, SHELTON R M, JIN Y, et al. Localized delivery of growth factors for periodontal tissue regeneration: role, strategies, and perspectives. Medicinal Research Reviews, 2009,29:472-513.
DOI URL |
| [5] | FIGLIUZZI M M, GIUDICE A, PILEGGI S, et al. Biomimetic hydroxyapatite used in the treatment of periodontal intrabony pockets: clinical and radiological analysis. Annali di Stomatologia, 2016,7(1/2):16-23. |
| [6] | SHEIKH Z, HAMDAN N, IKEDA Y, et al. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Biomaterials Research, 2017, 21: 9-1-20. |
| [7] |
WU C T, CHANG J. Silicate bioceramics for bone tissue regeneration. Journal of Inorganic Materials, 2013,28(1):29-39.
DOI URL |
| [8] |
ZHOU Y H, WU C T, XIAO Y. Silicate-based bioceramics for periodontal regeneration. Journal of Materials Chemistry B, 2014,2:3907-3910.
DOI URL |
| [9] |
ZHANG X F, HAN P P, JAIPRAKASH A, et al. Stimulatory effect of Ca3ZrSi2O9 bioceramics on cementogenic/osteogenic differentiation of periodontal ligament cells. Journal of Materials Chemistry B, 2014,2:1415-1423.
DOI URL |
| [10] |
ZHOU Y H, WU C T, ZHANG X F, et al. The ionic products from bredigite bioceramics induced cementogenic differentiation of periodontal ligament cells via activation of Wnt/β-catenin signalling pathway. Journal of Materials Chemistry B, 2013,1(27):3380-3389.
DOI URL |
| [11] |
ZHANG Y F, LI S, WU C T. The in vitro and in vivo cementogenesis of CaMgSi2O6 bioceramic scaffolds. Journal of Biomedical Materials Research A, 2014,102(1):105-116.
DOI URL |
| [12] | XIA L G, ZHANG Z Y, CHEN L, et al. Proliferation and osteogenic differentiation of human periodontalligament cells on akermanite and beta-TCP bioceramics. European Cells & Materials, 2011,22:68-83. |
| [13] |
ZHOU Y H, WU C T, XIAO Y. The stimulation of proliferation and differentiation of periodontal ligament cells by the ionic products from Ca7Si2P2O16 bioceramics. Acta Biomaterialia, 2012,8(6):2307-2316.
DOI URL |
| [14] |
WILLIAMS R S B, HARWOOD A J. Lithium therapy and signal transduction. Trends in Pharmacological Sciences, 2000,21(2):61-64.
DOI URL |
| [15] |
TANG L J, CHEN Y, PEI F X, et al. Lithium chloride modulates adipogenesis and osteogenesis of human bone marrow-derived mesenchymal stem cells. Cellular Physiology and Biochemistry, 2015,37(1):143-152.
DOI URL |
| [16] |
HEDGEPETH C M, CONRAD L J, ZHANG J, et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Developmental Biology, 1997,185:82-91.
DOI URL |
| [17] |
HAN P P, XU M C, CHANG J, et al. Lithium release from β-tricalcium phosphate inducing cementogenic and osteogenic differentiation for both hPDLCs and hBMSCs. Biomaterials Science, 2014,2:1230-1243.
DOI URL |
| [18] |
HAN P P, WU C T, CHANG J, et al. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials, 2012,33(27):6370-6379.
DOI URL |
| [19] |
PAN C H, CHEN L, WU R Y, et al. Lithium-containing biomaterials inhibiting osteoclastogenesis of macrophages in vitro and osteolysis in vivo. Journal of Materials Chemistry B, 2018,6:8115-8126.
DOI URL |
| [20] |
ZHAI D, CHEN L, CHEN Y, et al. Lithium silicate-based bioceramics promoting chondrocyte maturation by immunomodulating M2 macrophage polarization. Biomaterials Science, 2020,8:4521-4534.
DOI URL |
| [21] |
SOKOS D, EVERTS V, DE VRIES T J. Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. Journal of Periodontal Research, 2015,50(2):152-159.
DOI URL |
| [22] |
MCKEE M D, ADDISON W N, KAARTINEN M T. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs, 2005,181:176-188.
DOI URL |
| [23] |
MCKKEE M D, HOAC B, ADDISON W N, et al. Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontology 2000, 2013,63:102-122.
DOI URL |
| [24] |
LIU X Y, DING C X, CHU P K. Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials, 2004,25(10):1755-1761.
DOI URL |
| [25] |
HENSTOCK J R, CANH A M, ANDERSON S I. Silicon: the evolution of its use in biomaterials. Acta Biomaterials, 2015,11:17-26.
DOI URL |
| [26] |
ZHAI W Y, LU H X, WU C T, et al. Stimulatory effects of the ionic products from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomaterialia, 2013,9(8):8004-8014.
DOI URL |
| [27] | WU C T, CHEN Z T, YI D L, et al. Multidirectional effects of Sr, Mg and Si-containing bioceramic coatings with high bonding strength on inflammation, osteoclastogenesis and osteogenesis. ACS Applied Materials & Interfaces, 2014,6(6):4264-4276. |
| [28] |
ZHU H Y, ZHAI D, LIN C C, et al. 3D Plotting of highly uniform Sr5(PO4)2SiO4 bioceramic scaffolds for bone tissue engineering. Journal of Materials Chemistry B, 2016,4:6200-6212.
DOI URL |
| [29] |
STAHLI C, JAMES-BHASIN M, HOPPE A, et al. Effect of ion release from Cu-doped 45S5 Bioglass on 3D endothelial cell morphogenesis. Acta Biomaterialia, 2015,19:15-22.
DOI URL |
| [30] |
SEPULVEDA P, JONES J R, HENCH L L. In vitro dissolution of melt-derived 45S5 and Sol-Gel derived 58S bioactive glasses. Journal of Biomedical Materials Research, 2002,61:301-311.
DOI URL |
| [31] |
KAHLENBERG V, BRUNELLO E, HEJNY C, et al. Li2Ca2Si2O7: structural, spectroscopic and computational studies on a sorosilicate. Journal of Solid State Chemistry, 2015,225:155-167.
DOI URL |
| [32] | ZHANG J H, DUAN Y H, LIA C X. A first-principles investigation of structural properties, electronic structures and optical properties of β- and γ-LiAl (SiO3)2. Ceramics International, 2017,43(16):13948-13955. |
| [33] |
LARSSON L, DECKER A M, NIBALI L, et al. Regenerative medicine for periodontal and peri-implant diseases. Journal of Dental Research, 2016,95(3):255-266.
DOI URL |
| [34] |
BUNPETCH V, ZHANG X, LI T, et al. Silicate-based bioceramic scaffolds for dual-lineage regeneration of osteochondral defect. Biomaterials, 2019,192:323-333.
DOI URL |
| [1] | 蔡豪, 汪琦航, 邹朝勇. 镁离子调控无定形碳酸钙制备一水碳酸钙结晶过程[J]. 无机材料学报, 2024, 39(11): 1275-1282. |
| [2] | 吴锐, 张敏慧, 金成韵, 林健, 王德平. 光热核壳TiN@硼硅酸盐生物玻璃纳米颗粒的降解和矿化性能[J]. 无机材料学报, 2023, 38(6): 708-716. |
| [3] | 马磊, 黄毅, 邓浩, 银航, 田强, 晏敏皓. 氟磷灰石对酸性水溶液中铀(VI)的去除研究[J]. 无机材料学报, 2022, 37(4): 395-403. |
| [4] | 符明富, 杨雯, 李佳保, 邓书康, 周启航, 冯小波, 杨培志. 化学气相运输法制备正交黑磷[J]. 无机材料学报, 2022, 37(10): 1102-1108. |
| [5] | 朱子旻, 张敏慧, 张轩宇, 姚爱华, 林健, 王德平. 硼硅酸盐生物活性玻璃在直流电场下的体外矿化性能[J]. 无机材料学报, 2021, 36(9): 1006-1012. |
| [6] | 刘继涛, 钏定泽, 杨泽斌, 陈希亮, 颜廷亭, 陈庆华. 氨基酸/羟基磷灰石复合材料应用于酸蚀牛牙釉质体外再矿化[J]. 无机材料学报, 2019, 34(11): 1222-1230. |
| [7] | 董志红, 聂志萍, 周长春. 自然唾液中介孔生物活性玻璃诱导牙釉质仿生再矿化研究[J]. 无机材料学报, 2016, 31(1): 88-94. |
| [8] | 许金妹, 刘新玲, 高彦峰. 多巴胺辅助牙本质沉积羟基磷灰石研究[J]. 无机材料学报, 2016, 31(1): 95-99. |
| [9] | 鲁 元, 李京龙, 杨建锋, 李 鹏. 热处理温度对双连续β-氮化硅增强铝基复合材料性能的影响[J]. 无机材料学报, 2015, 30(3): 277-281. |
| [10] | 马玉菲, 乔 莉, 冯庆玲. 淡水珍珠的生物矿化机理研究进展[J]. 无机材料学报, 2013, 28(1): 109-116. |
| [11] | 朱云荣, 陈玉云, 许国华, 叶晓健, 钟 健, 何丹农. 丝素蛋白含量对纳米羟基磷灰石仿生矿化和体外细胞相容性的影响[J]. 无机材料学报, 2012, 27(8): 883-886. |
| [12] | 苏佳灿, 曹烈虎, 禹宝庆, 王志伟, 陈 晓, 李 明. 自固化硅羟基磷灰石骨水泥的制备及性能研究[J]. 无机材料学报, 2011, 26(1): 55-60. |
| [13] | 程 新, 李延报, 陆春华, 李东旭, 许仲梓. 预处理对构建丝素蛋白纤维/磷灰石仿生骨修复材料的影响[J]. 无机材料学报, 2011, 26(1): 43-48. |
| [14] | 杨 锦, 李君君, 袁欢欣, 欧阳健明. 氨羧钾调控草酸钙晶体生长及其与分子结构的关系[J]. 无机材料学报, 2010, 25(11): 1185-1190. |
| [15] | 熊浩洋,胡彬彬,薛中会,蔡 莉,戴树玺,杜祖亮. 牛血清蛋白单层分子膜诱导生长PbS晶体[J]. 无机材料学报, 2010, 25(1): 63-67. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||