无机材料学报 ›› 2021, Vol. 36 ›› Issue (12): 1337-1342.DOI: 10.15541/jim20210177 CSTR: 32189.14.10.15541/jim20210177
所属专题: 【虚拟专辑】碳中和(2020~2021)
田建建1,2( ), 马霞1,2, 王敏1, 姚鹤良1, 华子乐1, 张玲霞1,2,3(
), 马霞1,2, 王敏1, 姚鹤良1, 华子乐1, 张玲霞1,2,3( )
)
收稿日期:2021-03-19
修回日期:2021-05-07
出版日期:2021-12-20
网络出版日期:2021-05-25
通讯作者:
张玲霞, 研究员. E-mail: zhlingxia@mail.sic.ac.cn
作者简介:田建建(1989-), 女, 博士. E-mail: tianshujian11@163.com
TIAN Jianjian1,2( ), MA Xia1,2, WANG Min1, YAO Heliang1, HUA Zile1, ZHANG Lingxia1,2,3(
), MA Xia1,2, WANG Min1, YAO Heliang1, HUA Zile1, ZHANG Lingxia1,2,3( )
)
Received:2021-03-19
Revised:2021-05-07
Published:2021-12-20
Online:2021-05-25
Contact:
ZHANG Lingxia, professor. E-mail: zhlingxia@mail.sic.ac.cn
About author:TIAN Jianjian (1989-), female, PhD. E-mail: tianshujian11@163.com
Supported by:摘要:
锡基材料在自然界含量丰富、价格低廉, 在电催化还原CO2制液体燃料反应中具有巨大潜力。但是较低的产物选择性和较差的稳定性限制了其应用。本工作制备的锡量子点电催化剂(Sn-QDs), 具有高效、高稳定性和高选择性的电催化还原CO2产HCOOH活性。Sn-QDs的平均颗粒尺寸仅为2~3 nm, 结晶性良好。小的颗粒尺寸增大了电化学活性面积(ECSA), Sn-QDs的ECSA约为锡颗粒的4.4倍。ECSA增大以及CO2还原反应动力学加速, 促进了CO2电化学转化。在-1.0 V (vs RHE)下, Sn-QDs/CN催化剂的HCOOH法拉第效率(FEHCOOH)达到95%, 并且在宽约0.5 V的电势范围内能够保持在83%以上。此外, Sn-QDs/CN可以在24 h内保持良好的电化学稳定性。
中图分类号:
田建建, 马霞, 王敏, 姚鹤良, 华子乐, 张玲霞. 锡量子点制备及其电催化还原二氧化碳产甲酸性能[J]. 无机材料学报, 2021, 36(12): 1337-1342.
TIAN Jianjian, MA Xia, WANG Min, YAO Heliang, HUA Zile, ZHANG Lingxia. Sn Quantum Dots for Electrocatalytic Reduction of CO2 to HCOOH[J]. Journal of Inorganic Materials, 2021, 36(12): 1337-1342.
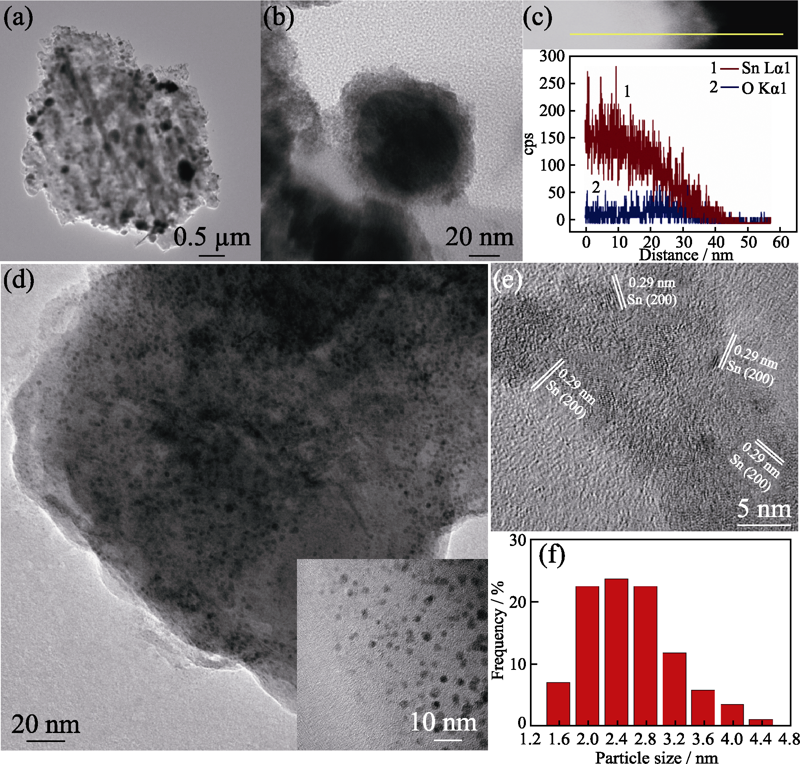
Fig. 2 TEM images at different magnifications (a, b) and corresponding EDS line scanning spectra (c) of Sn-p/CN; TEM image (inset: magnified image) (d), HRTEM image (e) and Sn-QDs size distribution (f) of Sn-QDs/CN
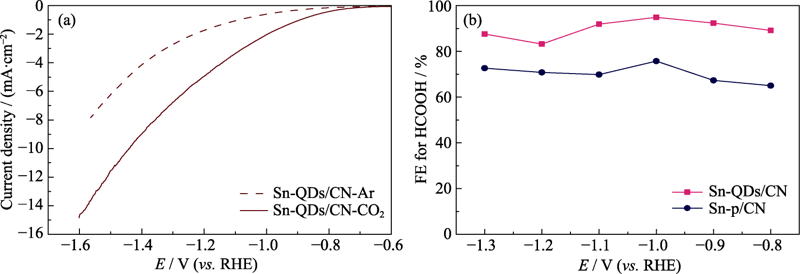
Fig. 4 LSV curves of the Sn-QDs/CN electrode in Ar-(dotted line) and CO2-saturated (solid line) 0.1 mol·L-1 KHCO3 electrolyte at a scan rate of 30 mV·s-1 (a), and Faradaic efficiencies of HCOOH on Sn-QDs/CN and Sn-p/CN at a series of potentials (b)
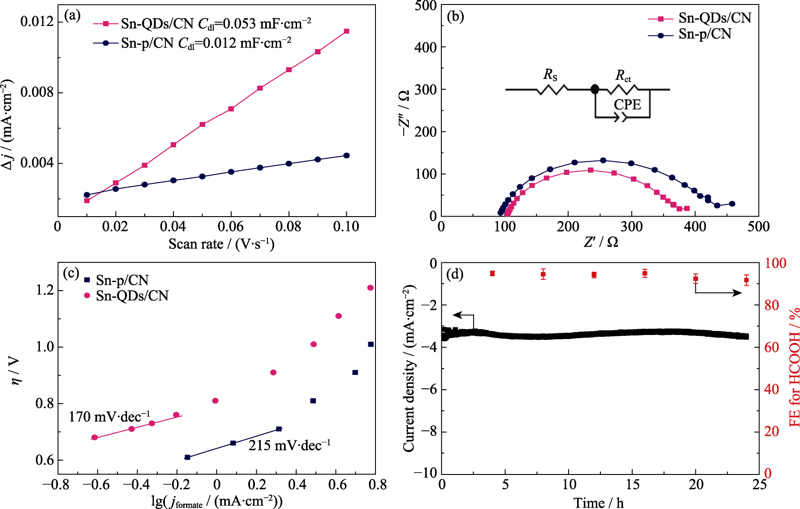
Fig. 5 Charging current density differences plotted against scan rates (a), electrochemical impedance spectra with inset showing the corresponding equivalent circuit (b), Tafel plots for HCOOH production on Sn-QDs/CN and Sn-p/CN (c), and the stability of Sn-QDs/CN catalyst at -1.0 V for 24 h in CO2-saturated 0.1 mol·L-1 KHCO3 (d)
| Electrocatalyst | Electrolyte | Potential /V (vs. RHE) | FEHCOOH /% | Current density /(mA·cm-2) | Stability/h | Ref. |
|---|---|---|---|---|---|---|
| Sn-QDs/CN | 0.1 mol·L-1 KHCO3 | -1.0 | 95 | 3.3 | 24 | This work |
| Sn quantum sheets confined in graphene | 0.1 mol·L-1 NaHCO3 | -1.2 | 89 | 21.1 | 50 | [1] |
| Nano-SnO2/graphene | 0.1 mol·L-1 NaHCO3 | -1.2 | 93.6 | 10 | - | [2] |
| SnO2 nanoparticles (< 5 nm) | 0.1 mol·L-1 KHCO3 | -1.2 | 64 | 147 | - | [3] |
| SnO2 nanoparticles (~500 nm) | 0.1 mol·L-1 KHCO3 | -1.2 | 83.5 | 7.56 | - | [4] |
| SnO2 nanoparticles (100 nm) | 0.5 mol·L-1 KHCO3 | -0.9 | 80 | 12 | - | [5] |
| SnO2 nanoparticles (8-20 nm) | 0.1 mol·L-1 KHCO3 | -1.06 | 82 | 15.3 | 5 | [6] |
| SnO2@N-CNW | 0.5 mol·L-1 NaHCO3 | -0.8 | 90 | 13 | 20 | [7] |
| SnO2@N-rGO | 0.5 mol·L-1 NaHCO3 | -0.8 | 89 | 21.3 | 20 | [8] |
| SnO2/PC | 0.5 mol·L-1 KHCO3 | -0.86 | 92 | 29 | 10 | [9] |
| SnO2⊃NC@EEG | 0.1 mol·L-1 KHCO3 | -1.2 | 81.2 | 13.4 | 10 | [10] |
| SnO/C | 0.5 mol·L-1 KHCO3 | -0.86 | 75 | 27.2 | - | [11] |
Table S1 Comparison of various Sn-based catalysts for CO2-to-HCOOH conversion
| Electrocatalyst | Electrolyte | Potential /V (vs. RHE) | FEHCOOH /% | Current density /(mA·cm-2) | Stability/h | Ref. |
|---|---|---|---|---|---|---|
| Sn-QDs/CN | 0.1 mol·L-1 KHCO3 | -1.0 | 95 | 3.3 | 24 | This work |
| Sn quantum sheets confined in graphene | 0.1 mol·L-1 NaHCO3 | -1.2 | 89 | 21.1 | 50 | [1] |
| Nano-SnO2/graphene | 0.1 mol·L-1 NaHCO3 | -1.2 | 93.6 | 10 | - | [2] |
| SnO2 nanoparticles (< 5 nm) | 0.1 mol·L-1 KHCO3 | -1.2 | 64 | 147 | - | [3] |
| SnO2 nanoparticles (~500 nm) | 0.1 mol·L-1 KHCO3 | -1.2 | 83.5 | 7.56 | - | [4] |
| SnO2 nanoparticles (100 nm) | 0.5 mol·L-1 KHCO3 | -0.9 | 80 | 12 | - | [5] |
| SnO2 nanoparticles (8-20 nm) | 0.1 mol·L-1 KHCO3 | -1.06 | 82 | 15.3 | 5 | [6] |
| SnO2@N-CNW | 0.5 mol·L-1 NaHCO3 | -0.8 | 90 | 13 | 20 | [7] |
| SnO2@N-rGO | 0.5 mol·L-1 NaHCO3 | -0.8 | 89 | 21.3 | 20 | [8] |
| SnO2/PC | 0.5 mol·L-1 KHCO3 | -0.86 | 92 | 29 | 10 | [9] |
| SnO2⊃NC@EEG | 0.1 mol·L-1 KHCO3 | -1.2 | 81.2 | 13.4 | 10 | [10] |
| SnO/C | 0.5 mol·L-1 KHCO3 | -0.86 | 75 | 27.2 | - | [11] |
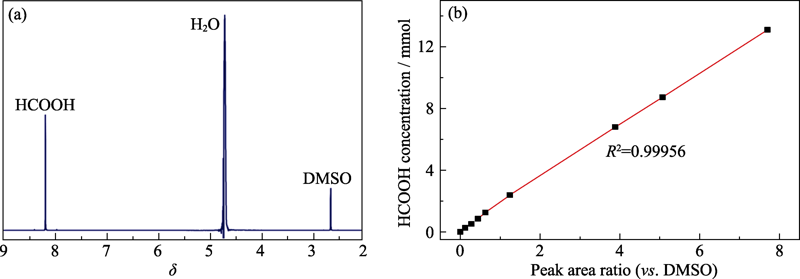
Fig. S1 1H NMR spectrum of the cathodic electrolyte after CO2RR (a), and linear relationship between HCOOH concentration and relative peak area ratio (vs. DMSO) (b)
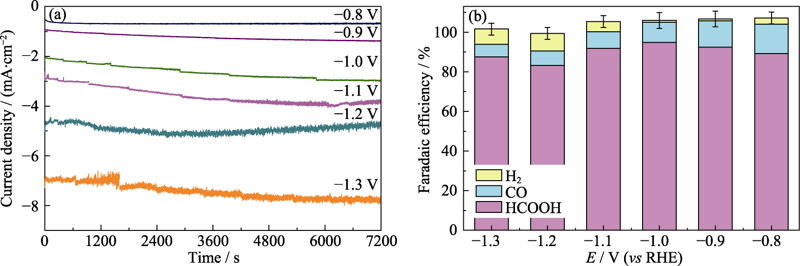
Fig. S3 i-t curves of CO2RR on Sn-QDs/CN at different applied potentials (a), and Faradaic efficiencies of HCOOH, CO and H2 at different applied potentials on the Sn-QDs/CN electrode (b)
| Parameter | Rs/Ω | Rct/Ω | CPE-T | CPE-P |
|---|---|---|---|---|
| Sn-QDs/CN | 102.6 | 276.3 | 1.2×10-5 | 0.85 |
| Sn-p/CN | 96.74 | 336.9 | 1.1×10-5 | 0.89 |
Table S2 Fitted data of EIS for Sn-QDs/CN and Sn-p/CN
| Parameter | Rs/Ω | Rct/Ω | CPE-T | CPE-P |
|---|---|---|---|---|
| Sn-QDs/CN | 102.6 | 276.3 | 1.2×10-5 | 0.85 |
| Sn-p/CN | 96.74 | 336.9 | 1.1×10-5 | 0.89 |
| [1] | LI X D, WANG S M, LI L, et al. Progress and perspective for in situ studies of CO2 reduction. Journal of the American Chemical Society, 2020, 142: 9567-9581. |
| [2] |
BIRDJA Y Y, PEREZ-GALLENT E, FIGUEIREDO M, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nature Energy, 2019, 4: 732-745.
DOI URL |
| [3] |
VASILEFF A, ZHENG Y, QIAO S Z. Carbon solving carbon's problems: recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2. Advanced Energy Materials, 2017, 7(21): 1700759.
DOI URL |
| [4] |
SHAO P, YI L C, CHEN S M, et al. Metal-organic frameworks for electrochemical reduction of carbon dioxide: the role of metal centers. Journal of Energy Chemistry, 2020, 40: 156-170.
DOI URL |
| [5] |
ZHANG L, ZHAO Z J, GONG J. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angewandte Chemie International Edition, 2017, 56(38): 11326-11353.
DOI URL |
| [6] |
MISTRY H, RESKE R, ZENG Z H, et al. Exceptional size- dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. Journal of the American Chemical Society, 2014, 136(47): 16473-16476.
DOI URL |
| [7] |
TYO E C, VAJDA S. Catalysis by clusters with precise numbers of atoms. Nature Nanotechnology, 2015, 10(7): 577-588.
DOI URL |
| [8] |
GAO D F, ZHOU H, WANG J, et al. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. Journal of the American Chemical Society, 2015, 137(13): 4288-4291.
DOI URL |
| [9] |
LIU S G, HUANG S P. Size effects and active sites of Cu nanoparticle catalysts for CO2 electroreduction. Applied Surface Science, 2019, 475: 20-27.
DOI URL |
| [10] |
LEE C H, KANAN M W. Controlling H+ vs CO2 reduction selectivity on Pb electrodes. ACS Catalysis, 2014, 5(1): 465-469.
DOI URL |
| [11] |
LI Z D, HE D, YAN X X, et al. Size-dependent nickel-based electrocatalysts for selective CO2 reduction. Angewandte Chemie International Edition, 2020, 59: 2-8.
DOI URL |
| [12] |
RESKE R, MISTRY H, BEHAFARID F, et al. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. Journal of the American Chemical Society, 2014, 136(19): 6978-6986.
DOI URL |
| [13] |
LÜ K L, SUO W Q, SHAO M D, et al. Nitrogen doped MoS2 and nitrogen doped carbon dots composite catalyst for electroreduction CO2 to CO with high Faradaic efficiency. Nano Energy, 2019, 63: 103834.
DOI URL |
| [14] |
LIU M, LIU M X, WANG X M, et al. Quantum-dot-derived catalysts for CO2 reduction reaction. Joule, 2019, 3(7): 1703-1718.
DOI URL |
| [15] |
WU J J, MA S C, SUN J, et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nature Communication, 2016, 7: 13869.
DOI URL |
| [16] | TIAN J J, WANG M, SHEN M, et al. Highly efficient and selective CO2 electro-reduction to HCOOH on Sn particle- decorated polymeric carbon nitride. ChemSusChem, 2020, 13(23): 6442-6448. |
| [17] |
WEN G B, LEE D U, REN B H, et al. Orbital interactions in Bi-Sn bimetallic electrocatalysts for highly selective electrochemical CO2 reduction toward formate production. Advanced Energy Materials, 2018, 8(31): 1802427.
DOI URL |
| [18] |
LI P X, FU W Z, ZHUANG P Y, et al. Amorphous Sn/crystalline SnS2 nanosheets via in situ electrochemical reduction methodology for highly efficient ambient N2 fixation. Small, 2019, 15(40): 1902535.
DOI URL |
| [19] |
TIAN J J, ZHANG L X, WANG M, et al. Remarkably enhanced H2 evolution activity of oxidized graphitic carbon nitride by an extremely facile K2CO3-activation approach. Applied Catalysis B: Environmental, 2018, 232: 322-329.
DOI URL |
| [20] |
WEN J, XIE J, CHEN X, et al. A review on g-C3N4-based photocatalysts. Applied Surface Science, 2017, 391: 72-123.
DOI URL |
| [21] |
LAI Q, YUAN W Y, HUANG W J, et al. Sn/SnOx electrode catalyst with mesoporous structure for efficient electroreduction of CO2 to formate. Applied Surface Science, 2020, 508: 145221.
DOI URL |
| [22] |
LIU S B, XIAO J, LU X F, et al. Efficient electrochemical reduction of CO2 to HCOOH over Sub-2 nm SnO2 quantum wires with exposed grain boundaries. Angewandte Chemie International Edition, 2019, 58: 8499-8503.
DOI URL |
| [23] |
LUC W, COLLINS C, WANG S W, et al. Ag-Sn bimetallic catalyst with a core-shell structure for CO2 reduction. Journal of the American Chemical Society, 2017, 139(5): 1885-1893.
DOI URL |
| [24] |
BANG J H, CHOI M S, MIRZAEI A, et al. Selective NO2 sensor based on Bi2O3 branched SnO2 nanowires. Sensors and Actuators B: Chemical, 2018, 274: 356-369.
DOI URL |
| [25] |
MA Y, WANG Z, XU X, et al. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chinese Journal of Catalysis, 2017, 38(12): 1956-1969.
DOI URL |
| [26] |
CHEN Z, GAO M R, DUAN N, et al. Tuning adsorption strength of CO2 and its intermediates on tin oxide-based electrocatalyst for efficient CO2 reduction towards carbonaceous products. Applied Catalysis B: Environmental, 2020, 277: 119252.
DOI URL |
| [27] |
NGUYEN T N, SALEHI M, LE Q, et al. Fundamentals of electrochemical CO2 reduction on single-metal-atom catalysts. ACS Catalysis, 2020, 10(17): 10068-10095.
DOI URL |
| [28] |
HE R, YUAN X, SHAO P F, et al. Hybridiztion of defective tin disulfide nanosheets and silver nanowires enables efficient electrochemical reduction of CO2 into formate and syngas. Small, 2019, 15(50): 1904882.
DOI URL |
| [1] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [2] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [3] | 李成金, 薛怡, 周晓霞, 陈航榕. BiZnx/Si光电阴极的制备及其CO2还原性能研究[J]. 无机材料学报, 2022, 37(10): 1093-1101. |
| [4] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [5] | 朱勇, 顾军, 于涛, 何海佟, 姚睿. 铂钴合金纳米电催化剂的制备及性能研究[J]. 无机材料学报, 2021, 36(3): 299-305. |
| [6] | 张清明, 朱敏, 周晓霞. CuO/ZnO复合电催化剂的制备及其还原CO2制合成气[J]. 无机材料学报, 2021, 36(11): 1145-1153. |
| [7] | 刘亚鑫, 王敏, 沈梦, 王强, 张玲霞. 铋掺杂提高氧化铈中氧空位浓度增强CO2光催化还原性能[J]. 无机材料学报, 2021, 36(1): 88-94. |
| [8] | 杨志宾, 岳彤联, 余向南, 吴苗苗. 钴掺杂氧化铈纳米粒子电催化性能研究[J]. 无机材料学报, 2018, 33(8): 845-853. |
| [9] | 张林森,王为,李振亚. Ni/AC膜电极-铝合金储氢电池的研究[J]. 无机材料学报, 2006, 21(5): 1103-1108. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||