无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1145-1153.DOI: 10.15541/jim20210092 CSTR: 32189.14.10.15541/jim20210092
所属专题: 【虚拟专辑】碳中和(2020~2021)
收稿日期:2021-02-13
修回日期:2021-03-30
出版日期:2021-11-20
网络出版日期:2021-03-15
通讯作者:
周晓霞, 副研究员. E-mail: zhouxiaoxia@mail.sic.ac.cn
作者简介:张清明(1995-), 男, 硕士研究生. E-mail: 18271648276@163.com
基金资助:
ZHANG Qingming1,2( ), ZHU Min1, ZHOU Xiaoxia2(
), ZHU Min1, ZHOU Xiaoxia2( )
)
Received:2021-02-13
Revised:2021-03-30
Published:2021-11-20
Online:2021-03-15
Contact:
ZHOU Xiaoxia, associate professor. E-mail: zhouxiaoxia@mail.sic.ac.cn
About author:ZHANG Qingming(1995-), male, Master candidate. E-mail: 18271648276@163.com
Supported by:摘要:
二氧化碳(CO2)还原制备合成气(CO和H2混合气), 不仅可以实现碳循环降低温室效应, 而且能缓解能源危机。而实现CO2资源化利用的关键在于催化剂设计。本研究采用金属离子共沉淀法制备了CuO及CuO/ZnO复合氧化物纳米材料, 通过调节催化剂组分, 探究其在不同电势下电化学CO2还原制备合成气的性能。结果表明: 引入锌(Zn)物种可以减弱中间物CO2•-在催化剂上的吸附强度, 导致CO的法拉第效率(FE)降低, 氢气FE增加, 从而实现不同电势下合成气CO/H2在1/1~1/4范围内的可控调节。尤其是, 当前驱液中铜和锌配比为1 : 2时, 在-0.9 V (vs. RHE)的电势下, CO和H2总FE高达84%。
中图分类号:
张清明, 朱敏, 周晓霞. CuO/ZnO复合电催化剂的制备及其还原CO2制合成气[J]. 无机材料学报, 2021, 36(11): 1145-1153.
ZHANG Qingming, ZHU Min, ZHOU Xiaoxia. CuO/ZnO Composite Electrocatalyst: Preparation and Reduction of CO2 to Syngas[J]. Journal of Inorganic Materials, 2021, 36(11): 1145-1153.

图3 催化剂(a, e) CuO、(b, f) CuO/ZnO-1、(c, g) CuO/ZnO-2和(d, h) CuO/ZnO-3在热解(a~d)前(e~h)后的TEM照片;催化剂CuO/ZnO-2的元素面扫分布图((i) TEM, (j) Cu, (k) O和(l) Zn)
Fig. 3 TEM images of catalysts (a, e) CuO, (b, f) CuO/ZnO-1, (c, g) CuO/ZnO-2 and (d, h) CuO/ZnO-3 (a-d) before and (e-h) after pyrolysis with elemental mapping images of CuO/ZnO-2 ((i) TEM, (j) Cu, (k) O and (l) Zn)
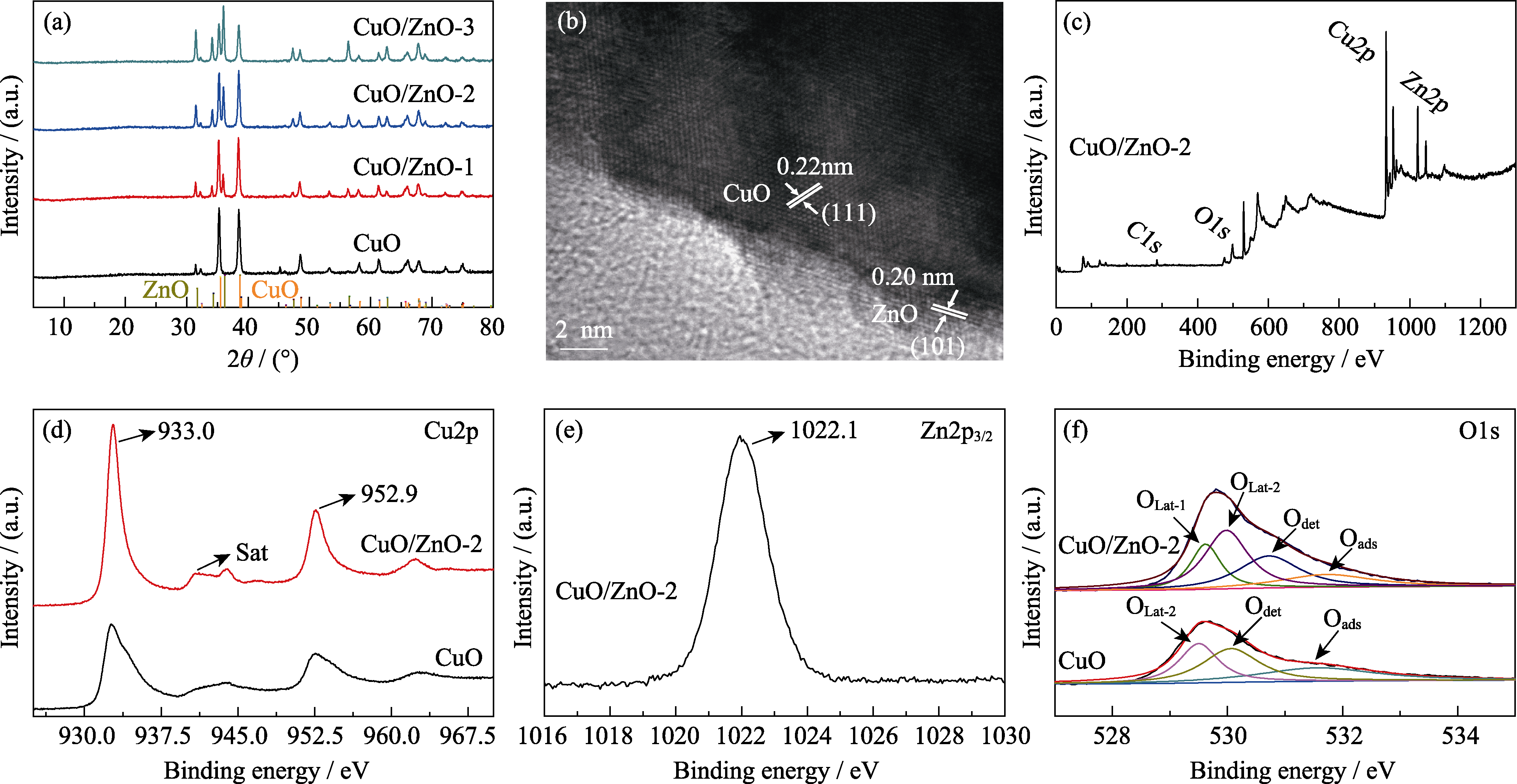
图4 (a) CuO、CuO/ZnO-1、CuO/ZnO-2和CuO/ZnO-3电催化剂的XRD图谱; (b) CuO/ZnO-2催化剂的HRTEM照片; (c) CuO/ZnO-2的XPS全谱谱图; (d) CuO/ZnO-2和CuO的Cu2p XPS分图; (e) CuO/ZnO-2的Zn2p3/2 XPS分图; (f) CuO/ZnO-2与CuO的XPS高分辨O1s谱图
Fig. 4 (a) XRD patterns of CuO, CuO/ZnO-1, CuO/ZnO-2 and CuO/ZnO-3 electrocatalysts, (b) HRTEM image of CuO/ZnO-2, (c) total XPS survey of CuO/ZnO-2, (d) XPS Cu2p spectra of CuO/ZnO-2 and CuO, (e) XPS Zn2p3/2 spectrum of CuO/ZnO-2, and (f) XPS high resolution O1s spectra of CuO/ZnO-2 and CuO Colorful figures are available on website
| Sample | Atomic percent/% | |||
|---|---|---|---|---|
| Cu | Zn | O | C | |
| CuO | 30.77 | 0 | 43.90 | 25.33 |
| CuO/ZnO-1 | 30.91 | 6.73 | 46.34 | 16.02 |
| CuO/ZnO-2 | 26.78 | 10.39 | 45.98 | 16.85 |
| CuO/ZnO-3 | 19.95 | 12.95 | 42.22 | 25.44 |
表1 样品表面元素含量分析
Table 1 Analysis of the surface elemental content
| Sample | Atomic percent/% | |||
|---|---|---|---|---|
| Cu | Zn | O | C | |
| CuO | 30.77 | 0 | 43.90 | 25.33 |
| CuO/ZnO-1 | 30.91 | 6.73 | 46.34 | 16.02 |
| CuO/ZnO-2 | 26.78 | 10.39 | 45.98 | 16.85 |
| CuO/ZnO-3 | 19.95 | 12.95 | 42.22 | 25.44 |
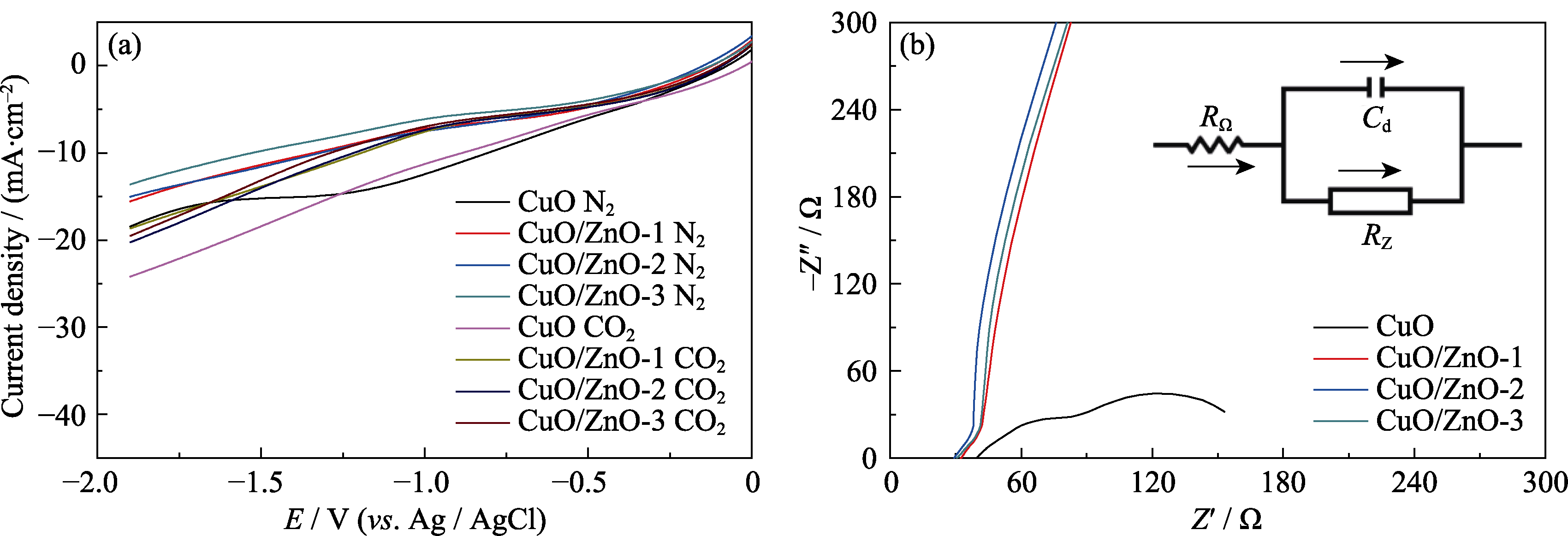
图5 CuO和CuO/ZnO催化剂(a)在N2/CO2饱和的KHCO3电解液中的线性扫描伏安曲线(LSVs)和(b)电化学阻抗谱图(EIS)
Fig. 5 (a) Linear sweep voltammetry curves (LSVs) in N2/CO2-saturated KHCO3, (b) electrochemical impedance spectra (EIS) of CuO and CuO/ZnO catalyst Colorful figures are available on website
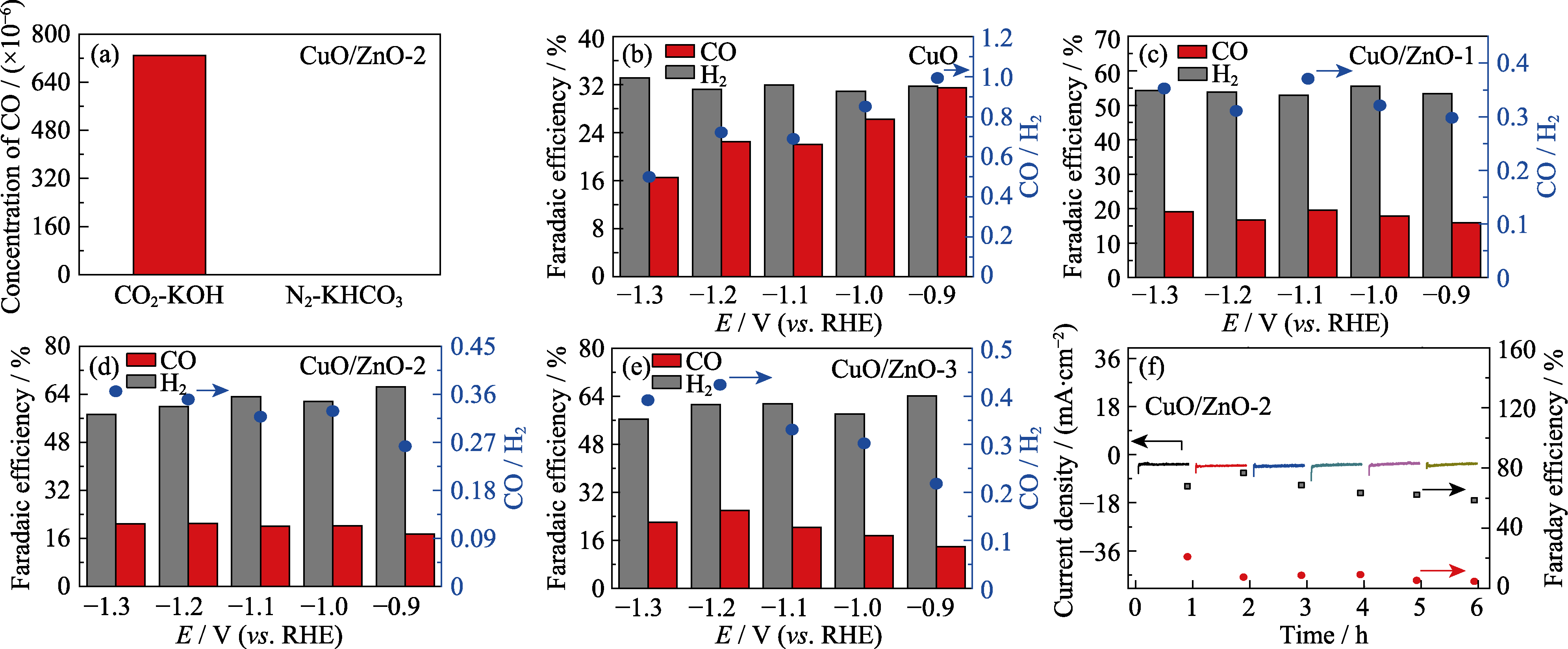
图6 (a) CuO/ZnO-2催化剂在CO2饱和的KOH溶液(CO2-KOH)和N2饱和的KHCO3溶液(N2-KHCO3)中CO2的还原活性; (b) CuO、(c) CuO/ZnO-1、(d) CuO/ZnO-2和(e) CuO/ZnO-3催化CO2还原产物的法拉第效率及对应的CO/H2的直方图; (f) CuO/ZnO-2的循环稳定性(黑色方形: H2法拉第效率, 红色圆点: CO法拉第效率)
Fig. 6 (a) Reduction activity of CO2 in CO2-saturated KOH solution (CO2-KOH) and N2-saturated KHCO3 solution (N2-KHCO3); Histograms for FE of the CO2 reduction products and the ratios of CO/H2 for (b) CuO, (c) CuO/ZnO-1, (d) CuO/ZnO-2 and (e) CuO/ ZnO-3; (f) Cyclic stability of CuO/ZnO-2 (black square and red dot indicate Faraday efficiencies of H2 and CO, respectively) Colorful figures are available on website
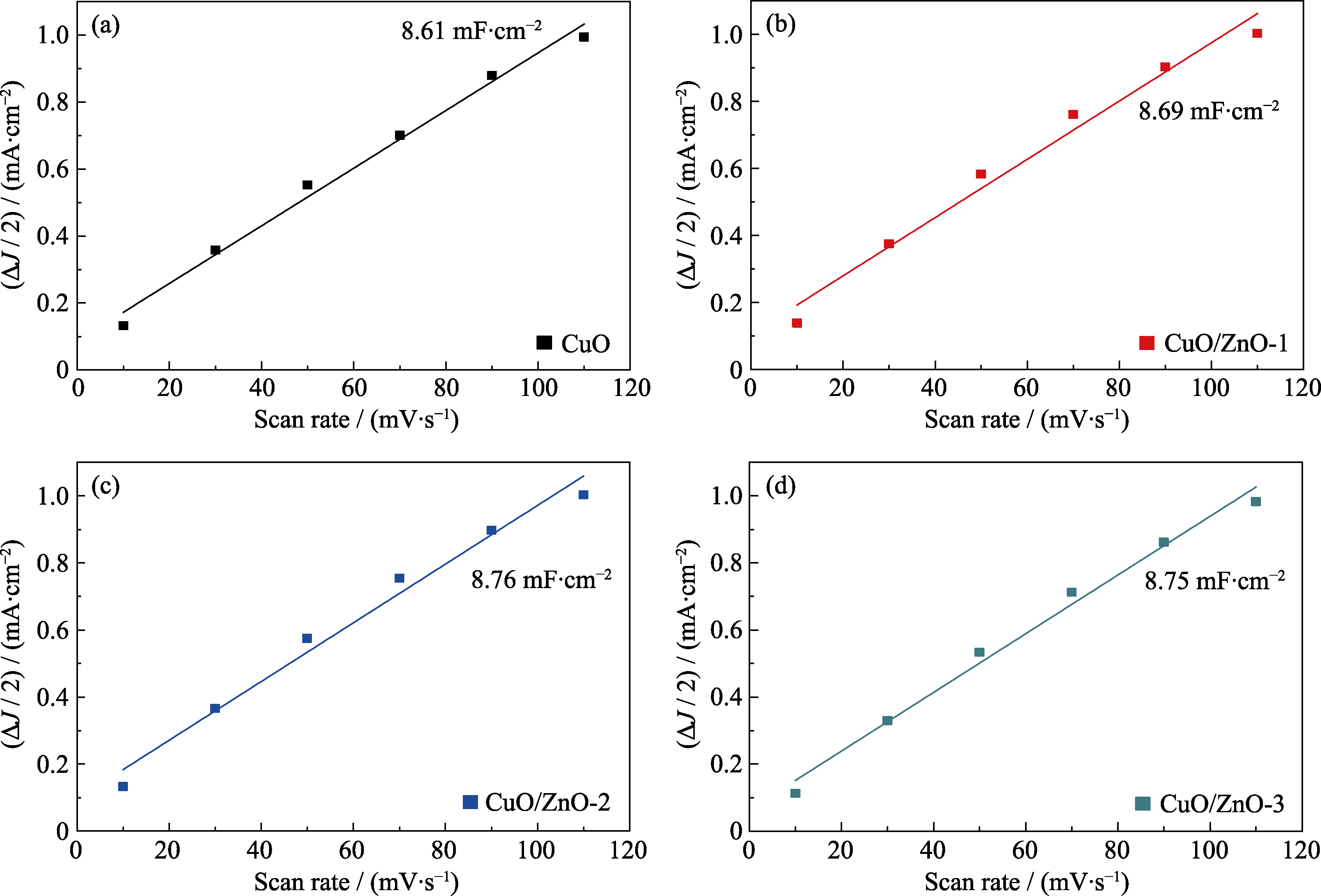
图7 (a) CuO、(b) CuO/ZnO-1、(c) CuO/ZnO-2和(d) CuO/ZnO-3电容ΔJ/2与扫描速率的线性拟合图
Fig. 7 Capacitive ΔJ/2 versus scan rate for (a) CuO, (b) CuO/ZnO-1, (c) CuO/ZnO-2, and (d) CuO/ZnO-3 Colorful figures are available on website
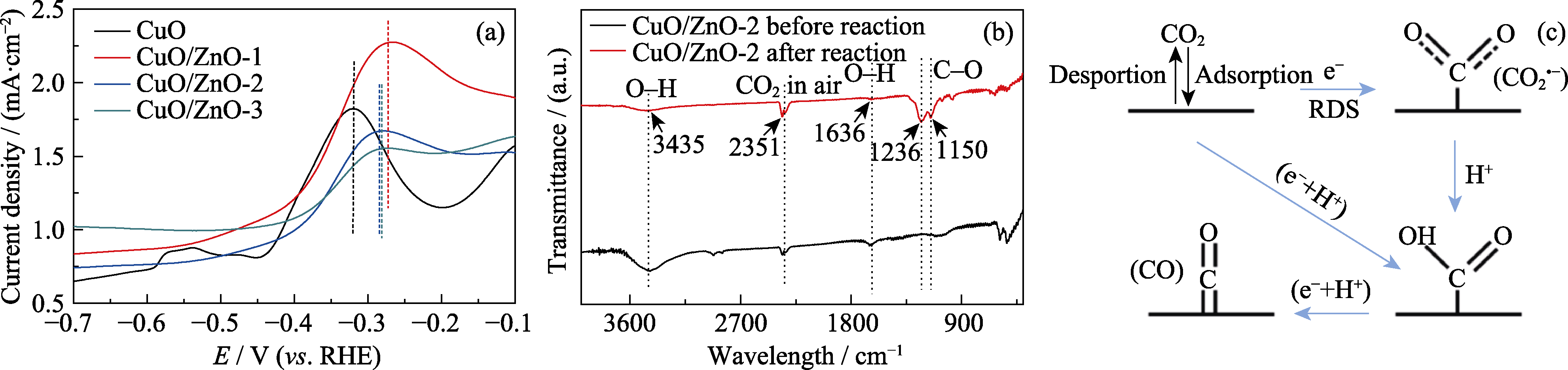
图8 (a) 在0.1 mol/L KOH溶液中, CuO、CuO/ZnO-1、CuO/ZnO-2和CuO/ZnO-3的氧化LSV曲线; (b) CuO/ZnO-2催化剂反应前后的红外光谱图; (c) CO的形成示意图
Fig. 8 (a) Oxidation LSVs of CuO, CuO/ZnO-1, CuO/ZnO-2 and CuO/ZnO-3 in 0.1 mol/L KOH; (b) Infrared spectra of the CuO/ZnO-2 catalyst before and after reaction; (c) Schematic formation process of CO Colorful figures are available on website
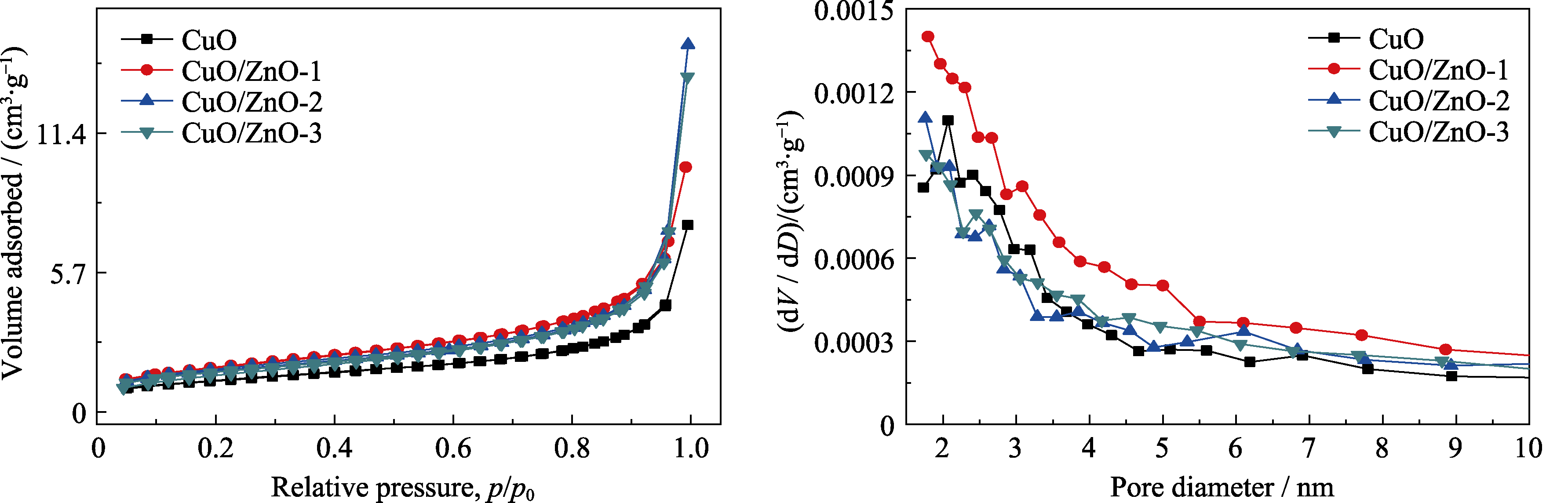
图S1 CuO、CuO/ZnO-1、CuO/ZnO-2和CuO/ZnO-3电催化剂的(a) N2吸脱附曲线和(b)孔径分布曲线
Fig. S1 (a) N2 adsorption/desorption curves and (b) pore size distributions of CuO, CuO/ZnO-1, CuO/ZnO-2 and CuO/ZnO-3 electrocatalysts
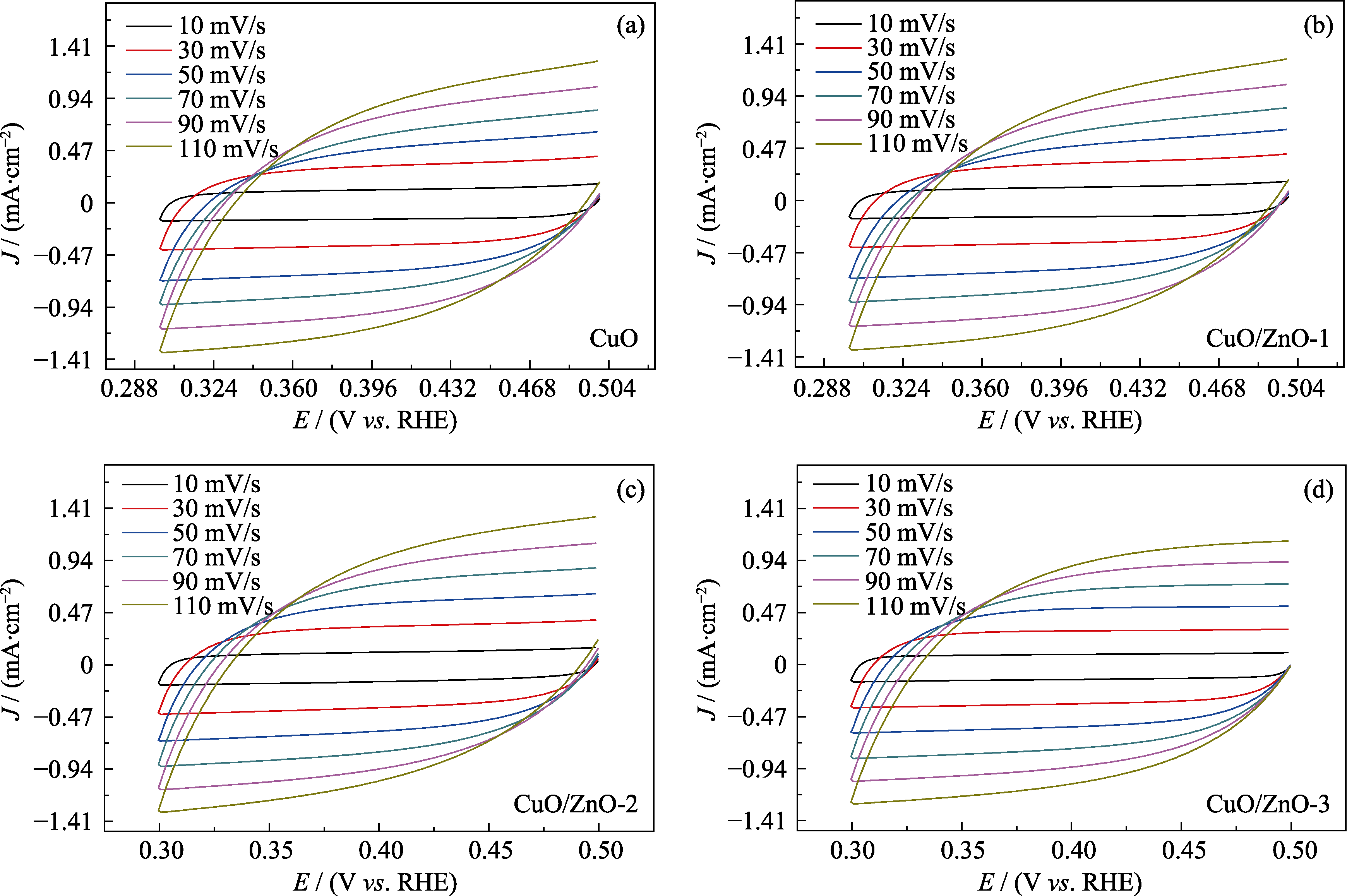
图S4 (a) CuO、(b) CuO/ZnO-1、(c) CuO/ZnO-2和(d) CuO/ZnO-3在不同扫描速率下的CV曲线
Fig. S4 (a) CV curves for (a) CuO, (b) CuO/ZnO-1, (c) CuO/ZnO-2 and (d) CuO/ZnO-3 at different scan rates
| [1] |
CHANG X X, WANG T, YANG P P, et al. The development of cocatalysts for photoelectrochemical CO2 reduction. Adv. Mater., 2019, 31(31):1804710.
DOI URL |
| [2] |
WU J H, HUANG Y, YE W, et al. CO2 reduction: from the electrochemical to photochemical approach. Adv. Sci., 2017, 4(11):1700194.
DOI URL |
| [3] |
LI X, YU J G, JARONIEC K, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev., 2019, 119(6):3962-4179.
DOI URL |
| [4] |
REN Y J, ZENG D Q, ONG W. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: a review. Chinese Journal of Catalysis, 2019, 40(3):289-319.
DOI URL |
| [5] |
MA S C, LIU J F, SASAKI K, et al. Carbon foam decorated with silver nanoparticles for electrochemical CO2 conversion. Energy Technology, 2017, 5(6):861-863.
DOI URL |
| [6] |
WU M F, ZHU C, WANG K, et al. Promotion of CO2 electrochemical reduction via Cu nanodendrites. ACS Appl. Mater. Interfaces, 2020, 12(10):11562-11569.
DOI URL |
| [7] |
CHU S, FAN S Z, WANG Y J, et al. Tunable syngas production from CO2 and H2O in an aqueous photoelectrochemical cell. Angew. Chem. Int. Ed., 2016, 55(46):14262-14266.
DOI URL |
| [8] |
CHU S, OU P F, RASHID R T, et al. Decoupling strategy for enhanced syngas generation from photoelectrochemical CO2 reduction. iScience, 2020, 23(8):101390.
DOI URL |
| [9] |
CHU S, OU P F, GHAMARI P, et al. Photoelectrochemical CO2 reduction into syngas with the metal/oxide interface. J. Am. Chem. Soc., 2018, 140(25):7869-7877.
DOI URL |
| [10] |
WOLDU A R. From low to high-index facets of noble metal nanocrystals: a way forward to enhance the performance of electrochemical CO2 reduction. Nanoscale, 2020, 12(16):8626-8635.
DOI URL |
| [11] |
TANG J, CHEN D, YAO Q F, et al. Recent advances in noble metal- based nanocomposites for electrochemical reactions. Materials Today Energy, 2017, 6:115-127.
DOI URL |
| [12] |
LI C Q, BAEK J B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega, 2020, 5(1):31-40.
DOI URL |
| [13] |
YANG Z N, OROPEZA F E, ZHANG K H L. P-block metal-based (Sn, In, Bi, Pb) electrocatalysts for selective reduction of CO2 to formate. APL Materials, 2020, 8(6):060901.
DOI URL |
| [14] | PÉREZ-RODRíGUEZ S, PASTOR E, LÁZARO M J. Noble metal- free catalysts supported on carbon for CO2 electrochemical reduction. Journal of CO2 Utilization, 2017, 18:41-52. |
| [15] |
BELL T E, TORRENTE-MURCIANO L. H2 production via ammonia decomposition using non-noble metal catalysts: a review. Topics in Catalysis, 2016, 59(15):1438-1457.
DOI URL |
| [16] |
WHITE L J, BARUCH M, PANDER J, et al. Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem. Rev., 2015, 115(23):12888-12935
DOI URL |
| [17] |
REN D, FONG J H, YEO B S. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun., 2018, 9(1):925.
DOI URL |
| [18] |
DAIYAN R, CHEN R, KUMAR P, et al. Tunable syngas production through CO2 electroreduction on cobalt-carbon composite electrocatalyst. ACS Appl. Mater. Interfaces, 2020, 12(8):9307-9315.
DOI URL |
| [19] |
SASTRE F, MUÑOZ-BATISTA M J, KUBACKA A, et al. Efficient electrochemical production of syngas from CO2 and H2O by using a nanostructured Ag/g-C3N4 catalyst. ChemElectroChem, 2016, 3(9):1497-1502.
DOI URL |
| [20] |
MA W C, XIE M C, XIE S J, et al. Nickel and indium core-shell co-catalysts loaded silicon nanowire arrays for efficient photoelectrocatalytic reduction of CO2 to formate. Journal of Energy Chemistry, 2021, 54:422-428.
DOI URL |
| [21] |
WANG Y H, LIU J L, WANG Y F, et al. Efficient solar-driven electrocatalytic CO2 reduction in a redox-medium-assisted system. Nat. Commun., 2018, 9(1):5003.
DOI URL |
| [22] |
ZHAO S K, SHEN Y B, HAO F L, et al. p-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Applied Surface Science, 2021, 538:148140.
DOI URL |
| [23] |
XIE Y, XING R Q, LI Q Q, et al. Three-dimensional ordered ZnO- CuO inverse opals toward low concentration acetone detection for exhaled breath sensing. Sensors and Actuators B: Chemical, 2015, 211:255-262.
DOI URL |
| [24] |
YE H C, NA W, GAO W G, et al. Carbon-modified CuO/ZnO catalyst with high oxygen vacancy for CO2 hydrogenation to methanol. Energy Technology, 2020, 8(6):2000194.
DOI URL |
| [25] |
ZHU L Y, LI H, LIU Z R, et al. Synthesis of the 0D/3D CuO/ZnO heterojunction with enhanced photocatalytic activity. The Journal of Physical Chemistry C, 2018, 122(17):9531-9539.
DOI URL |
| [26] |
WANG J J, WANG G J, ZHANG J F, et al. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angew. Chem. Int. Ed., 2021, 60(14):7602-7606.
DOI URL |
| [27] |
CHEN Z, FAN T T, ZHANG Y Q, et al. Wavy SnO2 catalyzed simultaneous reinforcement of carbon dioxide adsorption and activation towards electrochemical conversion of CO2 to HCOOH. Applied Catalysis B: Environmental, 2020, 261:118243.
DOI URL |
| [28] |
MOHAMMSDI A R, HABIBI Y A, BAYRAMI CHEN Z, et al. Enhanced anti-bacterial activities of ZnO nanoparticles and ZnO/ CuO nanocomposites synthesized using vaccinium arctostaphylos L. fruit extract. Artif. Cells Nanomed. Biotechnol., 2018, 46:1200-1209.
DOI URL |
| [29] |
CHEN F, ZHANG P P, XIAO L W, et al. Structure-performance correlations over Cu/ZnO interface for low-temperature methanol synthesis from syngas containing CO2. ACS Applied Mater Interfaces, 2021, 13(7):8191-8205.
DOI URL |
| [1] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [2] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [3] | 李成金, 薛怡, 周晓霞, 陈航榕. BiZnx/Si光电阴极的制备及其CO2还原性能研究[J]. 无机材料学报, 2022, 37(10): 1093-1101. |
| [4] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [5] | 田建建, 马霞, 王敏, 姚鹤良, 华子乐, 张玲霞. 锡量子点制备及其电催化还原二氧化碳产甲酸性能[J]. 无机材料学报, 2021, 36(12): 1337-1342. |
| [6] | 刘亚鑫, 王敏, 沈梦, 王强, 张玲霞. 铋掺杂提高氧化铈中氧空位浓度增强CO2光催化还原性能[J]. 无机材料学报, 2021, 36(1): 88-94. |
| [7] | 莫文龙, 马凤云, 刘月娥, 刘景梅, 钟 梅, 艾沙·努拉洪. 溶液燃烧法制备Ni-Al2O3催化剂用于CO2-CH4重整研究[J]. 无机材料学报, 2016, 31(5): 485-491. |
| [8] | 于建国, 王玉璋, 翁史烈. 煤气组分对固体氧化物燃料电池碳沉积的影响[J]. 无机材料学报, 2011, 26(11): 1129-1135. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||