无机材料学报 ›› 2018, Vol. 33 ›› Issue (6): 587-595.DOI: 10.15541/jim20170527 CSTR: 32189.14.10.15541/jim20170527
• • 下一篇
李汉超1,2,3, 刘盼盼1, 孙丽丽1, 柯培玲1, 崔平1,2, 汪爱英1
收稿日期:2017-11-09
修回日期:2017-12-25
出版日期:2018-06-20
网络出版日期:2018-05-24
作者简介:李汉超(1993-), 男, 博士研究生. E-mail: lihanchao@nimte.ac.cn
基金资助:LI Han-Chao1,2,3, LIU Pan-Pan1, SUN Li-Li1, KE Pei-Ling1, CUI Ping1,2, WANG Ai-Ying1
Received:2017-11-09
Revised:2017-12-25
Published:2018-06-20
Online:2018-05-24
About author:LI Han-Chao. E-mail: lihanchao@nimte.ac.cn
Supported by:摘要:
石墨烯自2004年被首次发现以来, 以其独特、优异的结构和特性引起了广泛关注。目前, 石墨烯的制备已取得了众多进展, 但在大尺寸、高质量、宏量石墨烯可控制备上仍存在挑战, 对制备技术仍需要进行更广泛地探索。非晶碳与石墨烯互为碳的同素异形体, 也可作为制备石墨烯的前驱体, 近些年利用非晶碳制备石墨烯的新颖方法引起了研究学者的兴趣。本文系统论述了利用非晶碳作为固体碳源, 通过金属催化制备大尺寸高质量石墨烯的技术优势, 并着重从金属种类、退火温度、碳源及金属含量比例等方面对石墨烯生成质量的影响进行了阐述。最后, 总结了该方法生长石墨烯的机理, 并对未来的发展方向进行了展望。
中图分类号:
李汉超, 刘盼盼, 孙丽丽, 柯培玲, 崔平, 汪爱英. 金属催化非晶碳转化制备石墨烯方法的研究进展[J]. 无机材料学报, 2018, 33(6): 587-595.
LI Han-Chao, LIU Pan-Pan, SUN Li-Li, KE Pei-Ling, CUI Ping, WANG Ai-Ying. Recent Development of the Transformation from Amorphous Carbon to Graphene Method via Metal Catalyst[J]. Journal of Inorganic Materials, 2018, 33(6): 587-595.
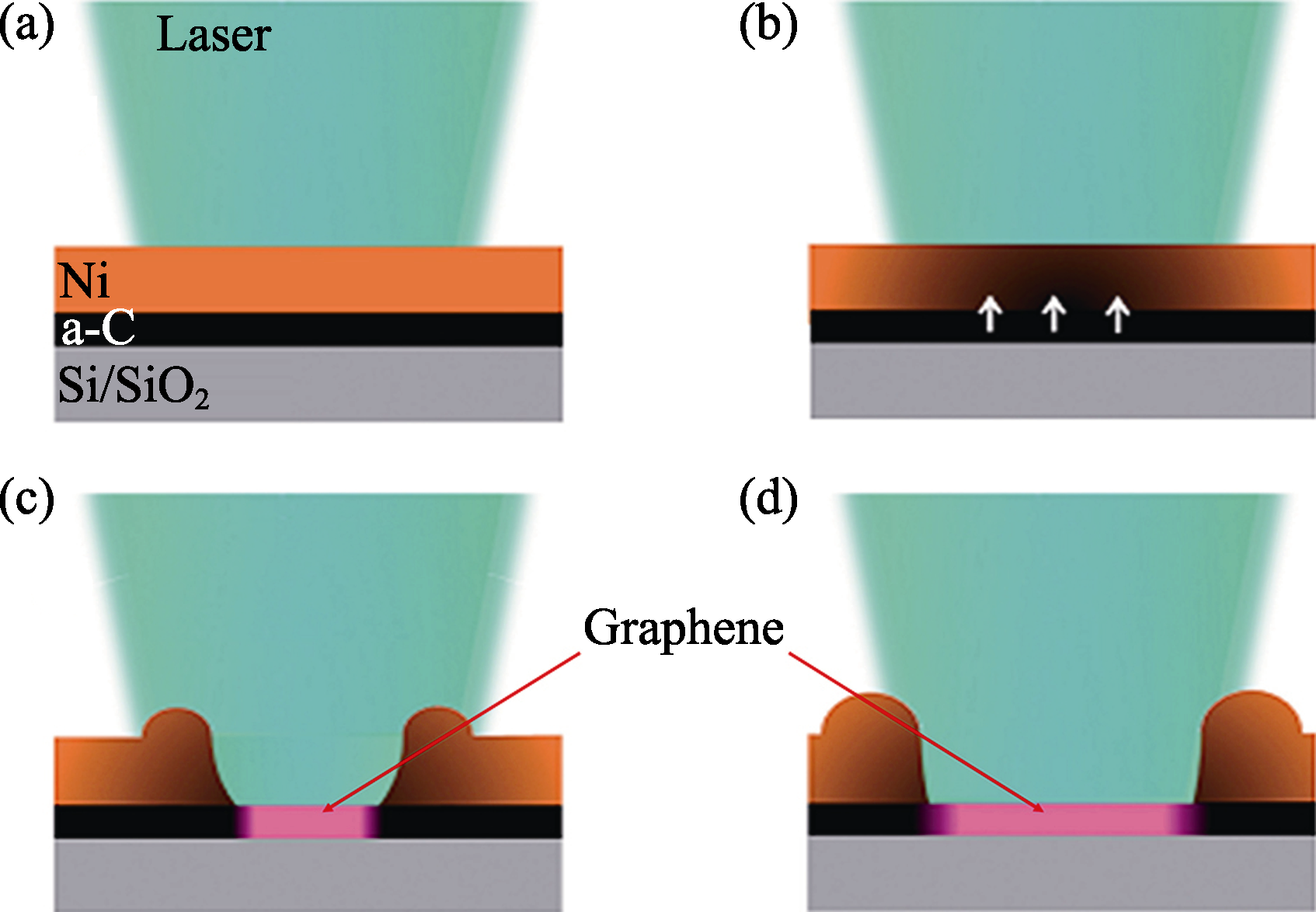
图1 激光照射下石墨烯形成原理图[30]
Fig. 1 Schematic of graphene synthesis mechanism[30](a) Local annealing of the Ni layer by laser irradiation; (b) Dissolution of a-C in the Ni layer; (c) Aggregation and retraction of the Ni layer; (d) Direct graphene synthesis on a SiO2 surface
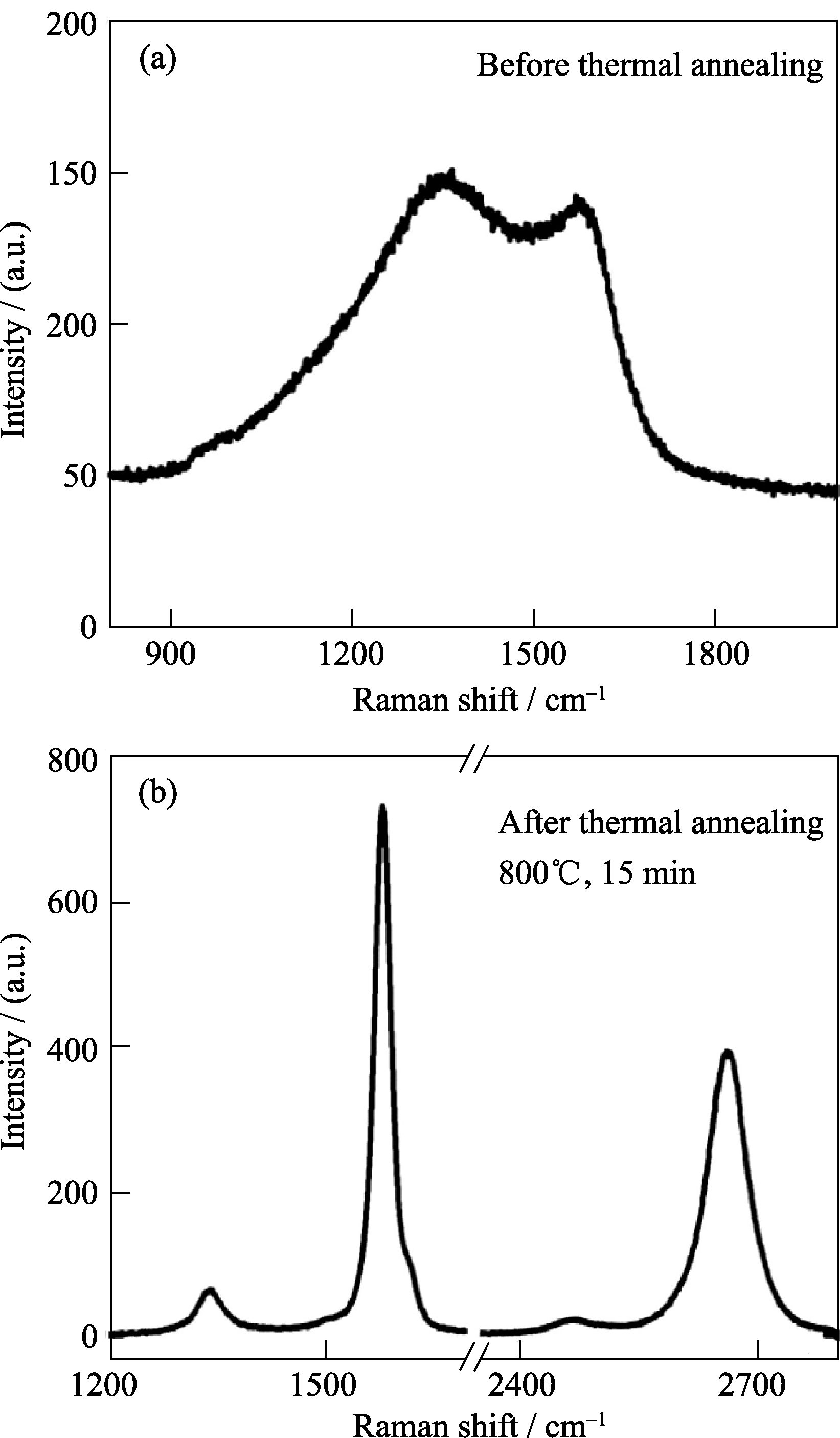
图5 (a) a-C(40 nm)和(b)Ni催化结晶后得到的石墨烯(Ni厚度~300 nm)的Raman光谱图, 激光发射波长632.8 nm[31]
Fig. 5 Raman spectra of (a) a-C (40 nm) and (b) the resulting graphene layer after Ni-catalyzed crystallization (Ni thickness ~300 nm) with excitation laser wavelength 632.8 nm[31]
| a-C method | T/℃ | Annealing time/min | Gas | Catalyst | Pressure/Pa | Graphene morphology | Raman | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| ID/IG | I2D/IG | ||||||||
| EBE | 650-950 | 15 | Ar | Ni, Co | 226 | Single, double layer | 0.09 | - | [31] |
| FVAD | 600-1000 | 5 | - | Ni | - | Few layer | - | - | [32] |
| PAPD | 700-1000 | 5 | N2 | Co, Ni | - | Multilayer | 0.59 | 1 | [35] |
| LA | 1000 | 30 | - | Ga | 1.33×10-2 | Few layer | - | - | [36] |
| FIB-CVD | 900-1100 | 30, 60 | - | Ga | - | Three, four layer | - | 0.84 | [37] |
| PLD | 800 | 5 | Ar | Co | 0.1 | Single, double layer | 2.5 | 0.67-1.43 | [39] |
| MS | 750-800 | 5-10 | - | Co, Ni | 3.0×10-4 | Single, double layer | - | 1.43 | [40] |
| DCMS | 1100 | 2 | Ar | Ni | 0.266 | Single, double layer | - | 2.5 | [46] |
| PAPD | 900 | 5 | N2 | Ni | - | Multilayer | - | - | [42] |
| DCMS | 900 | 30 | - | Ni | 2.66×10-4 | Single, double layer | 0.03 | 4.8 | [52] |
表1 退火热处理非晶碳转化石墨烯的实验参数及结果
Table 1 Parameters and results of thermal annealing for the experiment of transformation of a-C to graphene
| a-C method | T/℃ | Annealing time/min | Gas | Catalyst | Pressure/Pa | Graphene morphology | Raman | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| ID/IG | I2D/IG | ||||||||
| EBE | 650-950 | 15 | Ar | Ni, Co | 226 | Single, double layer | 0.09 | - | [31] |
| FVAD | 600-1000 | 5 | - | Ni | - | Few layer | - | - | [32] |
| PAPD | 700-1000 | 5 | N2 | Co, Ni | - | Multilayer | 0.59 | 1 | [35] |
| LA | 1000 | 30 | - | Ga | 1.33×10-2 | Few layer | - | - | [36] |
| FIB-CVD | 900-1100 | 30, 60 | - | Ga | - | Three, four layer | - | 0.84 | [37] |
| PLD | 800 | 5 | Ar | Co | 0.1 | Single, double layer | 2.5 | 0.67-1.43 | [39] |
| MS | 750-800 | 5-10 | - | Co, Ni | 3.0×10-4 | Single, double layer | - | 1.43 | [40] |
| DCMS | 1100 | 2 | Ar | Ni | 0.266 | Single, double layer | - | 2.5 | [46] |
| PAPD | 900 | 5 | N2 | Ni | - | Multilayer | - | - | [42] |
| DCMS | 900 | 30 | - | Ni | 2.66×10-4 | Single, double layer | 0.03 | 4.8 | [52] |
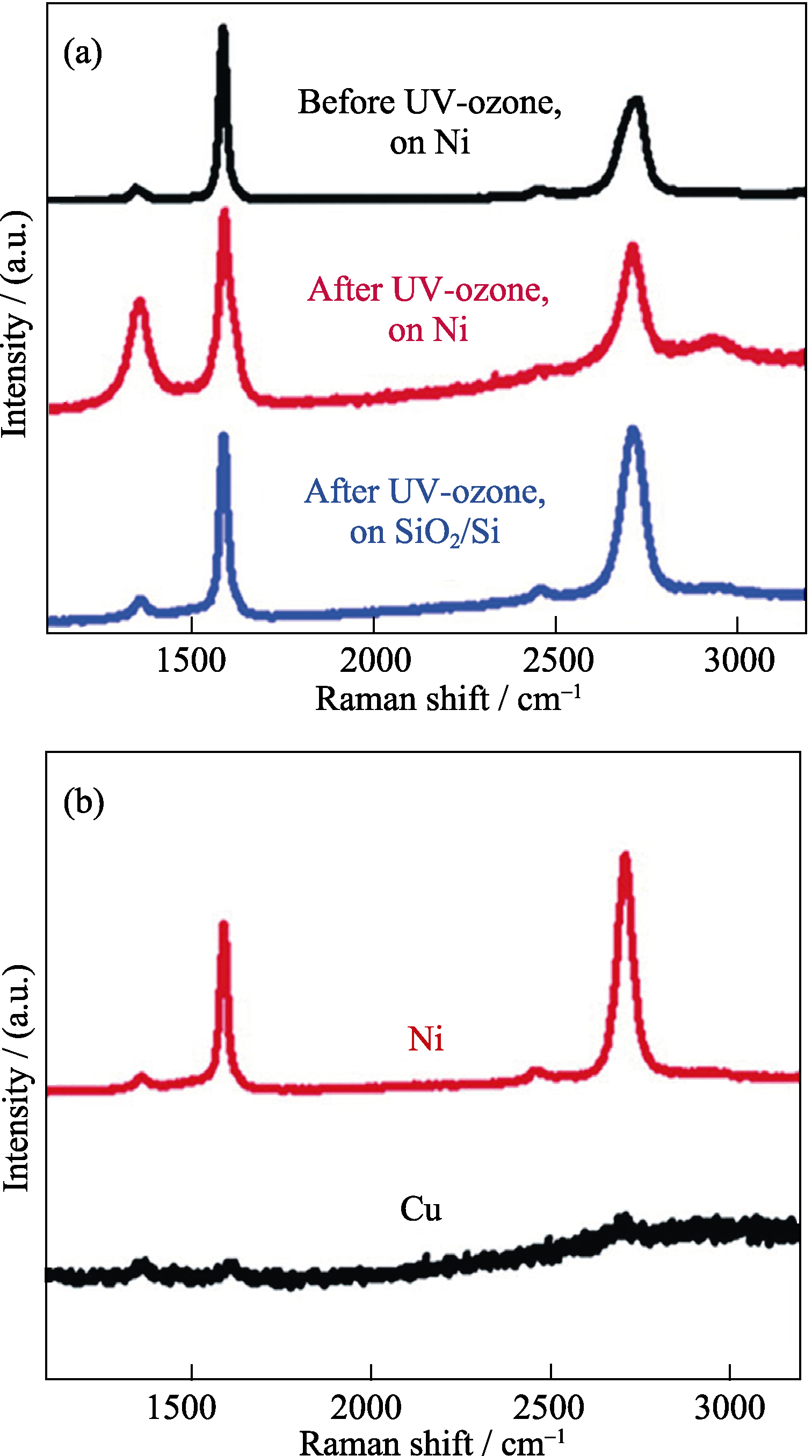
图6 不同生长条件下拉曼分析结果[33]
Fig. 6 Raman spectroscopic analysis of graphene from different growth conditions[33](a) Raman spectra of graphene on the top of the nickel layer before and after UV-ozone exposure, and graphene on the substrate after UV-ozone exposure and nickel removal; (b) Raman spectra of PMMA-derived graphene by different metal catalysts
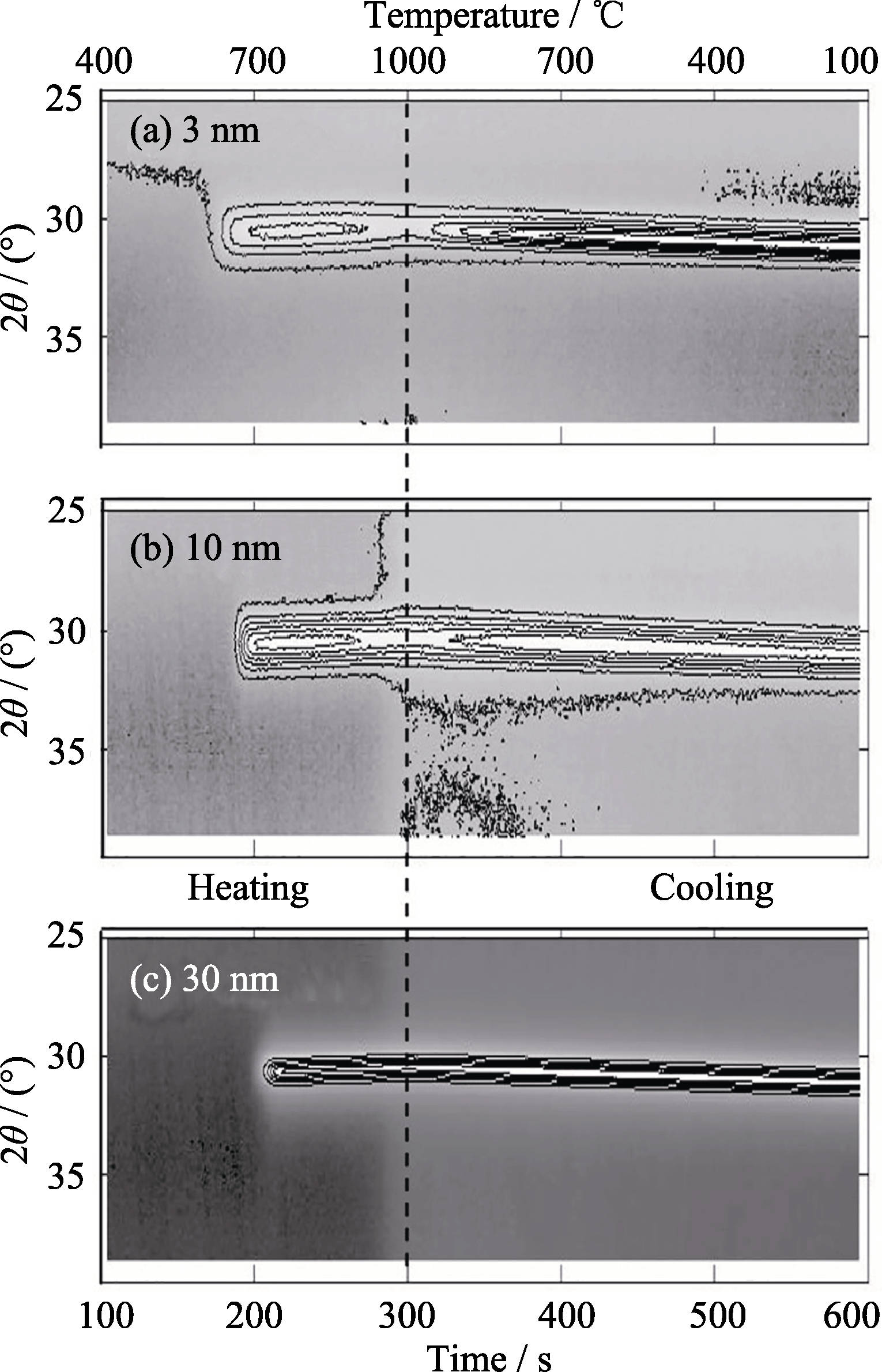
图7 不同厚度Si/SiO2/a-C/Ni(100 nm)样品在He气氛下加热至1000℃及冷却过程中原位XRD的(002)石墨峰强等高线图, 变温速率为3℃/s, 厚度(a) 3 nm、(b) 10 nm和(c) 30 nm。不同厚度a-C样品的等高线间隔不同[41]
Fig. 7 Contour maps of in situ XRD results showing the 002 graphite peak in Si/SiO2/a-C/Ni (100 nm) samples heated to and cooled from 1000℃ in He at a ramp rate of 3℃/s for a-C thicknesses of (a) 3 nm, (b) 10 nm, and (c) 30 nm. The contour lines have a linear intensity spacing that is different for each a-C thickness[41]
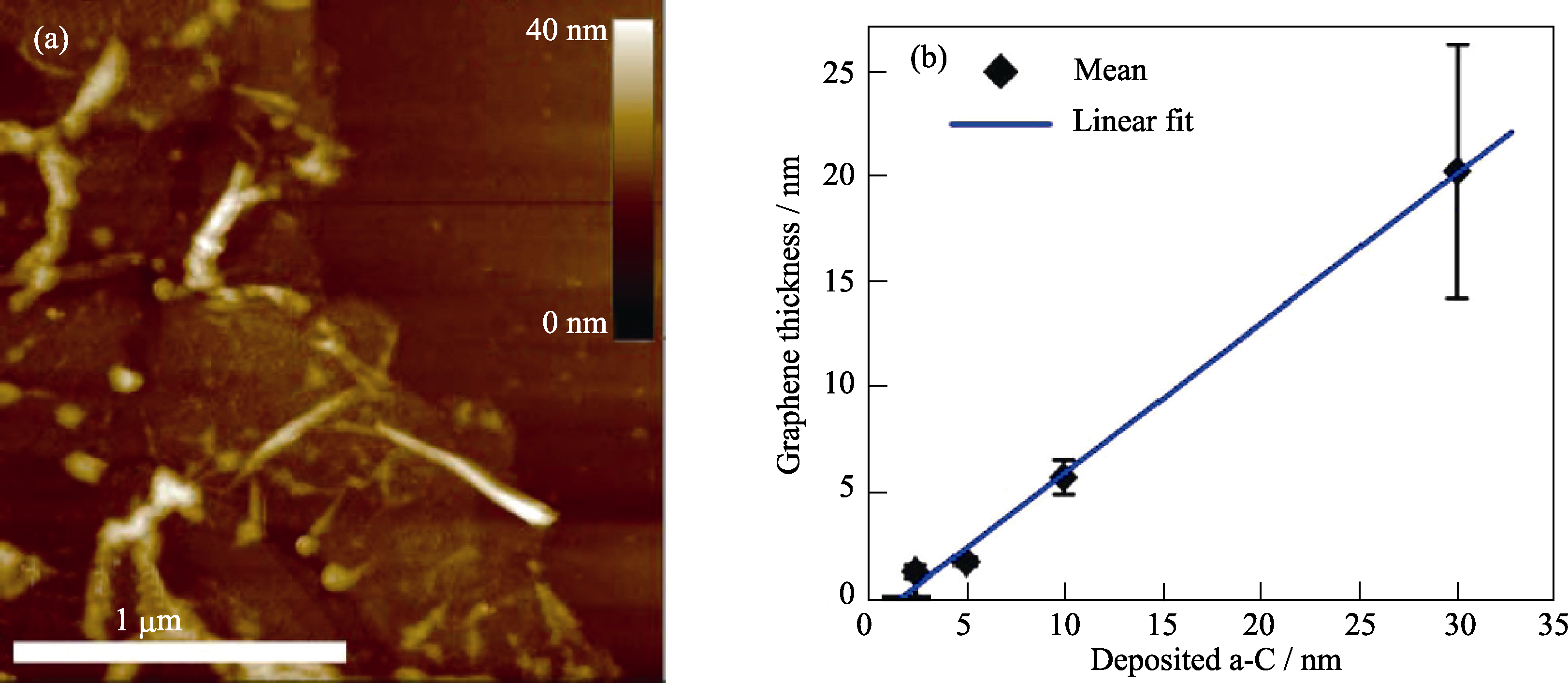
图8 (a)转移到Si/SiO2后的石墨烯AFM照片和(b)石墨烯厚度与原始a-C厚度的关系[31]
Fig. 8 (a) AFM image of a transferred graphene sheet on Si/SiO2 substrate; (b) Graphene (and graphite) thickness vs initial a-C thickness[31] Samples were annealed at 800?℃ for 15 min with a 300 nm Ni catalyst layer
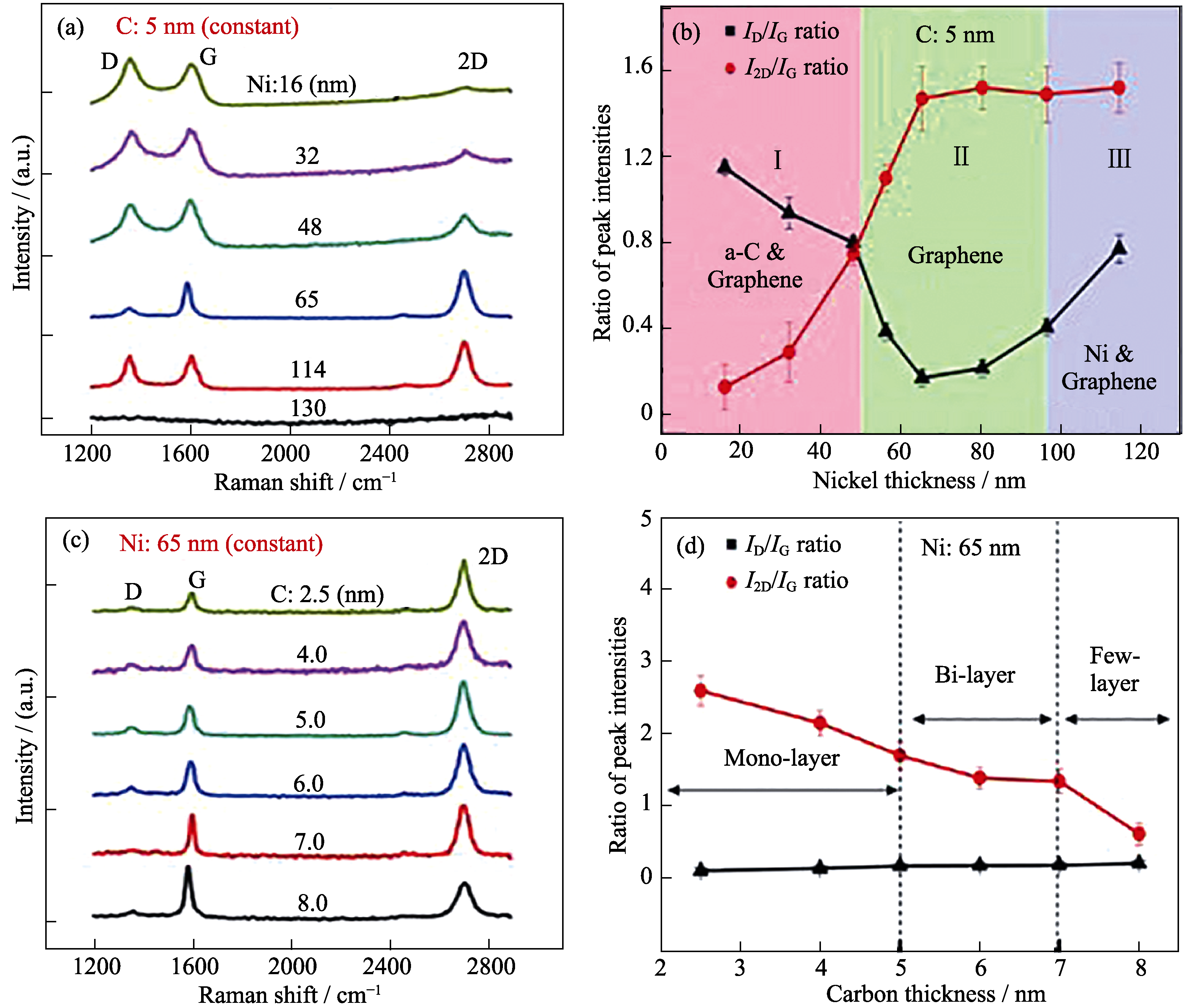
图9 a-C和Ni厚度对石墨烯生长的影响[46]
Fig. 9 Influence of Ni and C film thicknesses on graphene growth[46](a) Raman spectra of RTP graphene grown with a 5 nm C film covered with a Ni film of different thicknesses; (b) ID/IG and I2D/IG Raman peak ratios of the RTP graphene as functions of Ni film thickness; (c) Raman spectra of RTP graphene grown with a 65 nm Ni film on top of a C film with different thicknesses; (d) ID/IG and I2D/IG Raman peak ratios of the RTP graphene as functions of C film thickness
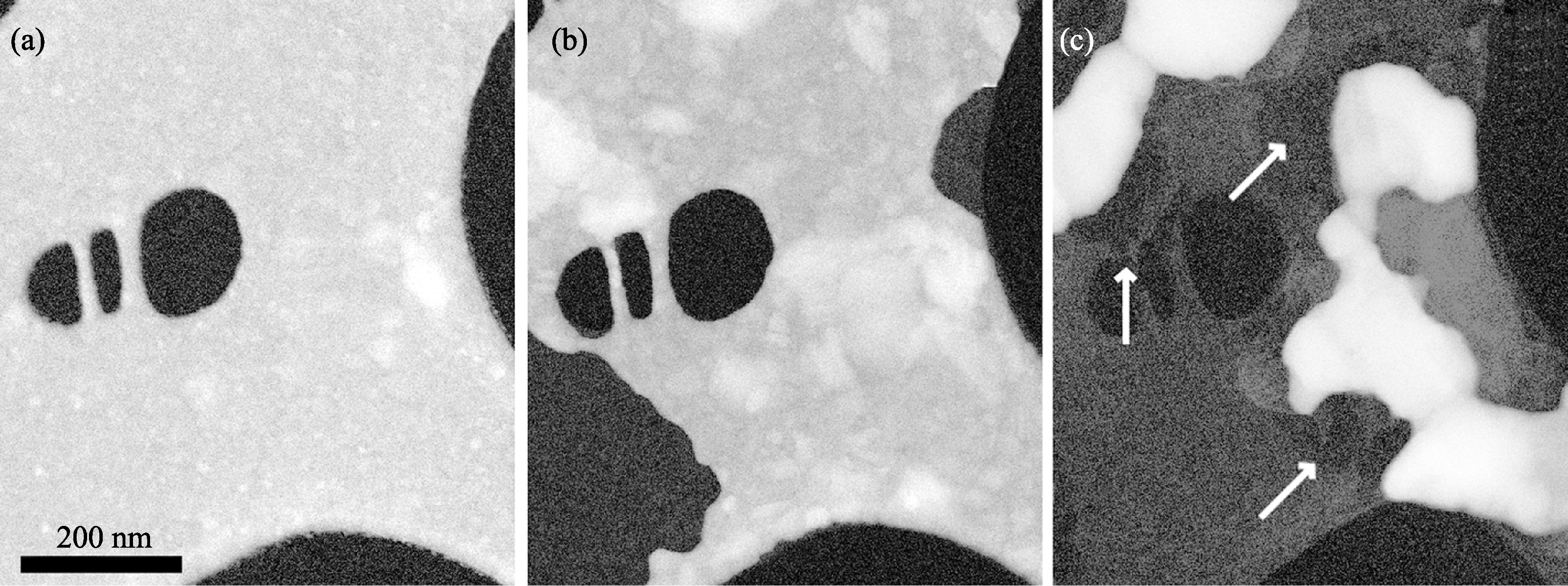
图10 非晶碳上Ni的暗场STEM照片[25]
Fig. 10 Plan-view scanning transmission electron microscopy (STEM) dark-field images of Ni crystals on an amorphous carbon film at 400℃ (a), 600℃ (b) and 720℃ (c) [25](a) At 400℃, the coherent polycrystalline Ni film (bright) covers the C substrate entirely; holes in the C film appear black; (b) At 600℃, ripening of the metal crystals starts and uncovers areas of the amorphous carbon film (light gray contrast); (c) At 720℃, ripening continues and graphene areas appear (dark, marked with arrows)
| [1] | BUNDY F P, HAll H T, STRONG H M,et al. Man-made diamonds. Nature, 1955, 176(4471): 51-55. |
| [2] | AISENBERG S, CHABOT R.Ion-beam deposition of thin films of diamondlike carbon. Journal of Applied Physics, 1971, 42(7): 2953-2958. |
| [3] | KROTO H W, HEATH J R, O'Brien S C,et al. C60: bucminsterfuleene. Nature, 1985, 318(6042): 162-163. |
| [4] | LIJIMA SUMIO.Helical microtubules of graphitic carbon.Nature, 1991, 354(6348): 56-58. |
| [5] | NOVOSELOV K S, GEIM A K, MOROZOV S V,et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696): 666-669. |
| [6] | LI G, LI Y, LIU H,et al. Architecture of graphdiyne nanoscale films. Chemical Communications, 2010, 46(19): 3256-3258. |
| [7] | LEE C, WEI X, KYSAR J W,et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321(5887): 385-388. |
| [8] | NAIR R R, BLAKE P, GRIGORENKO A N,et al. Fine structure constant defines visual transparency of graphene. Science, 2008, 320(5881): 1308. |
| [9] | BALANDIN A A, GHOSH S, BAO W,et al. Superior thermal conductivity of single layer graphene. Nano Letters, 2008, 8(3): 902-907. |
| [10] | MOROZOV S V, NOVOSELOV K S, KATSNELSON M I,et al. Giant intrisic carrier mobilities in graphene and its bilayer. Physical Review Letters, 2008, 100(1): 016602. |
| [11] | OHNO Y, MAEHASHI K, YAMASHIRO Y,et al. Electrolyte- gated graphene field-effect transistors for detecting pH and protein adsorption. Nano Letters, 2009, 9(9): 3318-3322. |
| [12] | YADAV P, BANERJEE A, UNNI S,et al. A 3D hexaporous carbon assembled from single-layer graphene as high performance supercapacitor. ChemSusChem, 2012, 5(11): 2159-2164. |
| [13] | LI S S, TU K H, LIN C C,et al. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano, 2010, 4(6): 3169-3174. |
| [14] | LIN Y M, DIMITRAKOPOULOS C, JENKINS K A,et al. 100-GHz transistors from wafer scale epitaxial graphene. Science, 2010, 327(5966): 662. |
| [15] | EMTSEV K V, BOSTWICK A, HORN K,et al. Towards wafer- size graphene layers by atmospheric pressure graphitization of silicon carbide. Nature Materials, 2009, 8(3): 203-207. |
| [16] | WARNER J H, SCHAFFEL F, RUMMELI M, et al. Graphene: Fundamentals and Emergent Applications. Boston: Newnes, 2012: 204-213. |
| [17] | PARK S, RUOFF R S.Chemical methods for the production of graphenes.Nature Nanotechnology, 2009, 4(4): 217-224. |
| [18] | STANKOVICH S, DIKIN D A, PINER R D,et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 2007, 45(7): 1558-1565. |
| [19] | LI X, CAI W, AN J,et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science, 2009, 324(5932): 1312-1314. |
| [20] | 任文才, 高力波, 马来鹏, 等. 石墨烯的化学气相沉积法制备. 新型碳材料, 2011, 26(01): 71-80. |
| [21] | 马来鹏, 任文才, 董再励, 等. 铜表面化学气相沉积石墨烯的研究进展: 生长行为与控制制备. 科学通报, 2012, 57(23): 2158-2163. |
| [22] | KANG J, SHIN D, BAE S,et al. Graphene transfer: key for applications. Nanoscale, 2012, 4(18): 5527-5537. |
| [23] | SUN Z, YAN Z, YAO J,et al. Growth of graphene from solid carbon sources. Nature, 2010, 468(7323): 549-552. |
| [24] | YAN Z, PENG Z, SUN Z,et al. Growth of bilayer graphene on insulating substrates. ACS Nano, 2011, 5(10): 8187-8192. |
| [25] | RODRIGUEZ-MANZO J A, PHAM-HUU C, BANHART F. Graphene growth by a metal catalyzed solid-state transformation of amorphous carbon.ACS Nano, 2011, 5(2): 1529-1534. |
| [26] | 王茂章, 杨全红, 成会明. 碳的结构及其同素异性体. 炭素技术, 2001(1): 23-28. |
| [27] | BAI L, ZHANG G, LU Z,et al. Tribological mechanism of hydrogenated amorphous carbon film against pairs: a physical description. Journal of Applied Physics, 2011, 110(3): 033521. |
| [28] | ROBERTSON J.Diamond-like amorphous carbon.Materials Science and Engineering: R: Reports, 2002, 37(4): 129-281. |
| [29] | DONNET C, ERDEMIR A.Historical developments and new trends in tribological and solid lubricant coatings.Surface and Coatings Technology, 2004, 180: 76-84. |
| [30] | KOSHIDA K, GUMI K, OHNO Y,et al. Position-controlled direct graphene synthesis on silicon oxide surfaces using laser irradiation. Applied Physics Express, 2013, 6(10): 105101. |
| [31] | ZHENG M, TAKEI K, HSIA B,et al. Metal-catalyzed crystallization of amorphous carbon to graphene. Applied Physics Letters, 2010, 96(6): 063110. |
| [32] | SEO J H, LEE H W, KIM J K,et al. Few layer graphene synthesized by filtered vacuum arc system using solid carbon source. Current Applied Physics, 2012, 12: 131-133. |
| [33] | PENG Z W, YAN Z, SUN Z Z,et al. Direct growth of bilayer graphene on SiO2 substrates by carbon diffusion through nickel. ACS Nano, 2011, 5(10): 8241-8247. |
| [34] | LI X, CAI W, COLOMBO L,et al. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Letters, 2009, 9(12): 4268-4272. |
| [35] | MIYOSHI M, MIZUNO M, BANNO K,et al. Study on transfer- free graphene synthesis process utilizing spontaneous agglomeration of catalytic Ni and Co metals. Materials Research Express, 2015, 2(1): 015602. |
| [36] | FUJITA J, UEKI R, MIYAZAWA Y,et al. Graphitization at interface between amorphous carbon and liquid gallium for fabricating large area graphene sheets. Journal of Vacuum Science & Technology B, 2009, 27(6): 3063-3066. |
| [37] | HATAKEYAMA T, KOMETANI R, WARISAWA S, et al. Selective graphene growth from DLC thin film patterned by focused- ion-beam chemical vapor deposition. Journal of Vacuum Science & Technology B. 2011, 29(6): 06FG04. |
| [38] | WANG J, CHEN L F, WU N,et al. Uniform graphene on liquid metal by chemical vapour deposition at reduced temperature. Carbon, 2016, 96: 799-804. |
| [39] | HIRANO R, MATSUBARA K, KALITA G,et al. Synthesis of transfer-free graphene on an insulating substrate using a solid phase reaction. Nanoscale, 2012, 4(24): 7791-7796. |
| [40] | OROFEO C M, AGO H, HU B,et al. Synthesis of large area, homogeneous, single layer graphene films by annealing amorphous carbon on Co and Ni. Nano Research, 2011, 4(6): 531-540. |
| [41] | SAENGER K L, TSANG J C, BOL A A,et al. In situ X-ray diffraction study of graphitic carbon formed during heating and cooling of amorphous-C/Ni bilayers. Applied Physics Letters, 2010, 96(15): 153105. |
| [42] | BANNO K, MIZUNO M, FUJITA K,et al. Transfer-free graphene synthesis on insulating substrates via agglomeration phenomena of catalytic nickel films. Applied Physics Letters, 2013, 103(8): 082112. |
| [43] | CHU J H, KWAK J, KWON T Y,et al. Facile synthesis of few- layer graphene with a controllable thickness using rapid thermal annealing. ACS Applied Materials & Interfaces, 2012, 4(3): 1777-1782. |
| [44] | WENISCH R, HÜBNER R, MUNNIK F,et al. Nickel-enhanced graphitic ordering of carbon ad-atoms during physical vapor deposition. Carbon, 2016, 100: 656-663. |
| [45] | ANTON R.On the reaction kinetics of Ni with amorphous carbon.Carbon, 2008, 46(4): 656-662. |
| [46] | XIONG W, ZHOU Y S, JIANG L J,et al. Single-step formation of graphene on dielectric surfaces. Advanced Materials, 2013, 25(4): 630-634. |
| [47] | LENG Y, XIE L, LIAO F,et al. Kinetic and thermodynamics studies on the decompositions of Ni3C in different atmospheres. Thermochimica Acta, 2008, 473(1): 14-18. |
| [48] | KOVÁCS G J, BERTÓTI I, RADNÓCZI G. X-ray photoelectron spectroscopic study of magnetron sputtered carbon-nickel composite films.Thin Solid Films, 2008, 516(21): 7942-7946. |
| [49] | ASAKA K, SAITO Y.Spontaneous graphenization of amorphous carbon on clean surfaces of nanometer-sized nickel particles at room temperature.Carbon, 2016, 103: 352-355. |
| [50] | KWAK J, CHU J H, CHOI J K,et al. Near room-temperature synthesis of transfer-free graphene films. Nature Communications, 2012, 3: 645. |
| [51] | 刘盼盼, 李汉超, 杨林等. 退火温度对金属催化四面体非晶碳转变石墨过程的影响. 材料研究学报, 2018. DOI:10.11901/1005.3093.2017.107. |
| [52] | NGUYEN B S, LIN J F, PERNG D C.Non-vacuum growth of graphene films using solid carbon source.Applied Physics Letters, 2015, 106(22): 221604. |
| [53] | CHEN Y Z, MEDINA H, LIN H C,et al. Large-scale and patternable graphene: direct transformation of amorphous carbon film into graphene/graphite on insulators via Cu mediation engineering and its application to all-carbon based devices. Nanoscale, 2015, 7(5): 1678-1687. |
| [54] | SCHNEIDER J J.Transforming amorphous into crystalline carbon: observing how graphene grows.ChemCatChem, 2011, 3(7): 1119-1120. |
| [55] | 张朝华, 付磊, 张艳锋, 等. 石墨烯催化生长中的偏析现象及其调控方法. 化学学报, 2013, 71(03): 308-322. |
| [1] | 杨茗凯, 黄泽皑, 周芸霄, 刘彤, 张魁魁, 谭浩, 刘梦颖, 詹俊杰, 陈国星, 周莹. 基于Cu与金属氧化物-KCl熔融介质的甲烷热解制备少层石墨烯与氢气联产研究[J]. 无机材料学报, 2025, 40(5): 473-480. |
| [2] | 高晨光, 孙晓亮, 陈君, 李达鑫, 陈庆庆, 贾德昌, 周玉. 基于湿法纺丝技术的SiBCN-rGO陶瓷纤维的组织结构、力学和吸波性能[J]. 无机材料学报, 2025, 40(3): 290-296. |
| [3] | 王悦, 王欣, 于显利. 室温铁磁性还原氧化石墨烯基全碳膜[J]. 无机材料学报, 2025, 40(3): 305-313. |
| [4] | 李红兰, 张俊苗, 宋二红, 杨兴林. Mo/S共掺杂的石墨烯用于合成氨: 密度泛函理论研究[J]. 无机材料学报, 2024, 39(5): 561-568. |
| [5] | 孙川, 何鹏飞, 胡振峰, 王荣, 邢悦, 张志彬, 李竞龙, 万春磊, 梁秀兵. 含有石墨烯阵列的SiC基陶瓷材料的制备与力学性能[J]. 无机材料学报, 2024, 39(3): 267-273. |
| [6] | 王艳莉, 钱心怡, 沈春银, 詹亮. 石墨烯基介孔锰铈氧化物催化剂: 制备和低温催化还原NO[J]. 无机材料学报, 2024, 39(1): 81-89. |
| [7] | 杨平军, 李铁虎, 李昊, 党阿磊. 石墨烯对环氧树脂泡沫炭石墨化、电导率和力学性能的影响[J]. 无机材料学报, 2024, 39(1): 107-112. |
| [8] | 董怡曼, 谭占鳌. 宽带隙钙钛矿基二端叠层太阳电池复合层的研究进展[J]. 无机材料学报, 2023, 38(9): 1031-1043. |
| [9] | 陈赛赛, 庞雅莉, 王娇娜, 龚䶮, 王锐, 栾筱婉, 李昕. 绿-黄可逆电热致变色织物的制备及其性能[J]. 无机材料学报, 2022, 37(9): 954-960. |
| [10] | 孙铭, 邵溥真, 孙凯, 黄建华, 张强, 修子扬, 肖海英, 武高辉. RGO/Al复合材料界面性质第一性原理研究[J]. 无机材料学报, 2022, 37(6): 651-659. |
| [11] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [12] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [13] | 董淑蕊, 赵笛, 赵静, 金万勤. 离子化氨基酸对氧化石墨烯膜渗透汽化过程中水选择性渗透的影响[J]. 无机材料学报, 2022, 37(4): 387-394. |
| [14] | 蒋丽丽, 徐帅帅, 夏宝凯, 陈胜, 朱俊武. 缺陷调控石墨烯复合催化剂在氧还原反应中的作用[J]. 无机材料学报, 2022, 37(2): 215-222. |
| [15] | 吴静, 余立兵, 刘帅帅, 黄秋艳, 姜姗姗, ANTON Matveev, 王连莉, 宋二红, 肖蓓蓓. NiN4/Cr修饰的石墨烯电化学固氮电极[J]. 无机材料学报, 2022, 37(10): 1141-1148. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||