
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (11): 1163-1170.DOI: 10.15541/jim20210109
• RESEARCH ARTICLE • Previous Articles Next Articles
GUO Yu( ), JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin
), JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin
Received:2021-02-23
Revised:2021-04-26
Published:2021-11-20
Online:2021-04-30
About author:GUO Yu(1981-), male, professor. E-mail: guoyu@lnut.edu.cn
Supported by:CLC Number:
GUO Yu, JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin. Preparation of 2-hydroxy-1-naphthalene Functionalized SBA-15 Adsorbent for the Adsorption of Chromium(III) Ions from Aqueous Solution[J]. Journal of Inorganic Materials, 2021, 36(11): 1163-1170.
| Sample | SBET/(cm2·g-1) | Pore size/nm |
|---|---|---|
| SBA-15 | 757 | 9.6 |
| Q-SBA-15 | 283 | 6.7 |
Table 1 Textural properties of the synthesized samples
| Sample | SBET/(cm2·g-1) | Pore size/nm |
|---|---|---|
| SBA-15 | 757 | 9.6 |
| Q-SBA-15 | 283 | 6.7 |
| Temperature /℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm/(g·mg-1) | KL | R2 | KF | n | R2 | ||
| 30 | 106.84 | 0.09 | 0.988 | 24.59 | 3.12 | 0.836 | |
| 40 | 108.23 | 0.25 | 0.997 | 48.39 | 5.47 | 0.913 | |
| 50 | 112.74 | 0.41 | 0.997 | 42.72 | 4.27 | 0.697 | |
Table 2 Different model parameters for adsorption of Cr(III) by Q-SBA-15 at different temperatures
| Temperature /℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm/(g·mg-1) | KL | R2 | KF | n | R2 | ||
| 30 | 106.84 | 0.09 | 0.988 | 24.59 | 3.12 | 0.836 | |
| 40 | 108.23 | 0.25 | 0.997 | 48.39 | 5.47 | 0.913 | |
| 50 | 112.74 | 0.41 | 0.997 | 42.72 | 4.27 | 0.697 | |
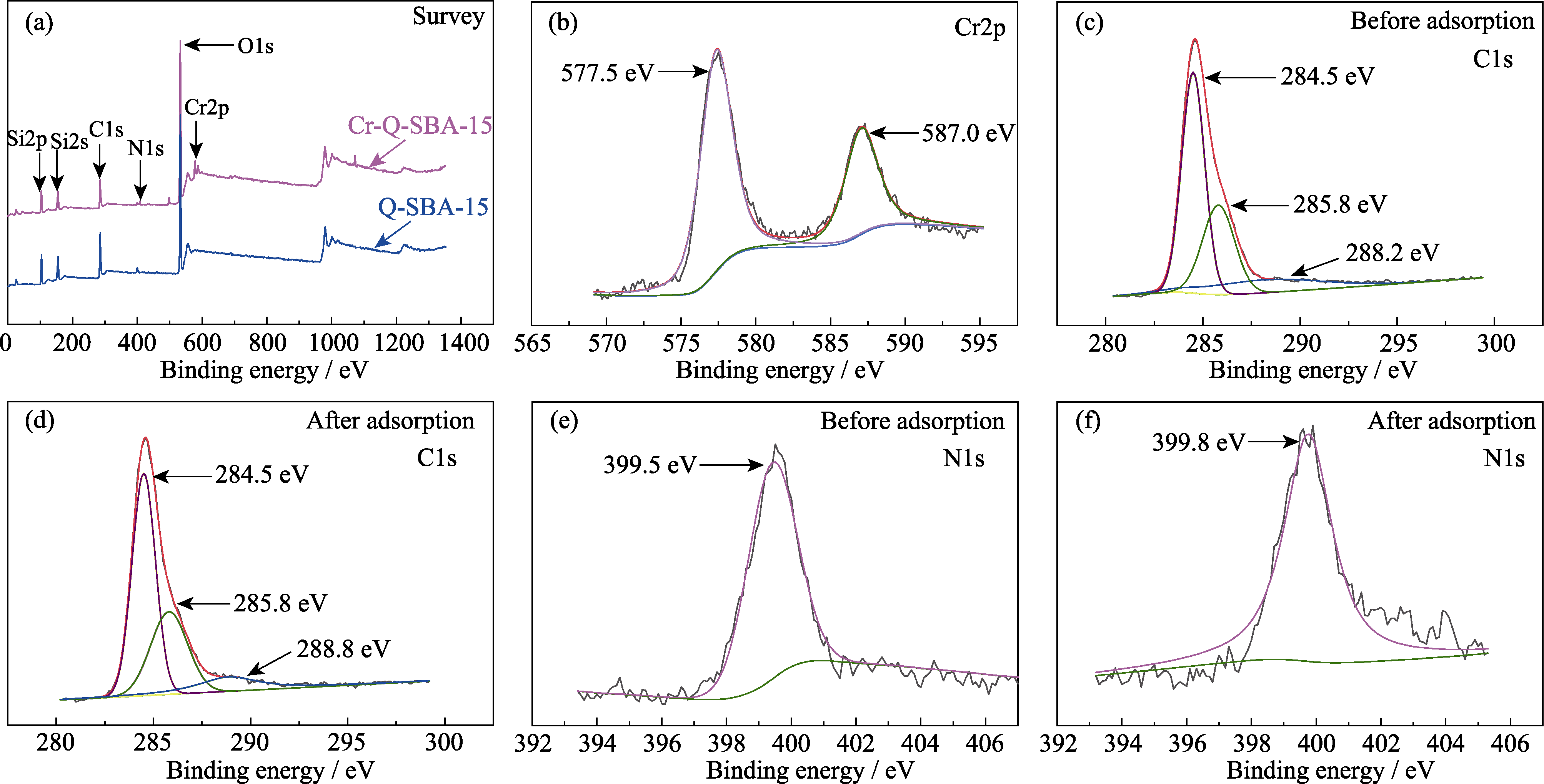
Fig. 8 XPS spectra of Q-SBA-15 (a) Overview spectra of Q-SBA-15 before and after Cr(III) adsorption; (b) Cr2p spectrum of Q-SBA-15 after Cr(III) adsorption; C1s spectra of (c) Q-SBA-15 (before adsorption) and (d) Q-SBA-15 (after adsorption); N1s spectra of (e) Q-SBA-15 (before adsorption) and (f) Q-SBA-15 (after adsorption)
| Sample | qe,exp/(mg·g-1) | Pseudo first-order model | Pseudo second-order model | ||||
|---|---|---|---|---|---|---|---|
| K1/ min-1 | qe,cal/(mg·g-1) | R2 | K2/(g·mg-1·min-1) | qe,cal/(mg·g-1) | R2 | ||
| Q-SBA-15 | 102.3 | 0.0647 | 61.75 | 0.857 | 4.26×10-4 | 119.19 | 0.979 |
Table S1 Kinetic parameters for Cr(III) adsorption onto Q-SBA-15
| Sample | qe,exp/(mg·g-1) | Pseudo first-order model | Pseudo second-order model | ||||
|---|---|---|---|---|---|---|---|
| K1/ min-1 | qe,cal/(mg·g-1) | R2 | K2/(g·mg-1·min-1) | qe,cal/(mg·g-1) | R2 | ||
| Q-SBA-15 | 102.3 | 0.0647 | 61.75 | 0.857 | 4.26×10-4 | 119.19 | 0.979 |
| Adsorbent | qm/(mg·g-1) | References |
|---|---|---|
| Biomass-based hydrogel | 41.7 | [1] |
| m-MCM-41-NH2 | 36.92 | [2] |
| Graphene oxide | 13.3 | [3] |
| Thiol-functionalized MCM-41 | 15.34 | [4] |
| TS-SBA-15 | 95.68 | [5] |
| NH2-SBA-15 | 24.88 | [6] |
| Q-SBA-15 | 102.3 | This work |
Table S2 Comparison of Cr(III) adsorption performance with different materials selected from literature
| Adsorbent | qm/(mg·g-1) | References |
|---|---|---|
| Biomass-based hydrogel | 41.7 | [1] |
| m-MCM-41-NH2 | 36.92 | [2] |
| Graphene oxide | 13.3 | [3] |
| Thiol-functionalized MCM-41 | 15.34 | [4] |
| TS-SBA-15 | 95.68 | [5] |
| NH2-SBA-15 | 24.88 | [6] |
| Q-SBA-15 | 102.3 | This work |
| Sample | Tempera- ture/K | Kc | ΔG/ (kJ·mol-1) | ΔS/ (kJ·mol·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|---|---|
| Q-SBA-15 | 293 | 1.846 | -4.386 | 0.128 | 33.118 |
| 303 | 2.095 | -5.666 | |||
| 313 | 2.650 | -6.946 | |||
| 318 | 2.780 | -7.586 | |||
| 323 | 3.088 | -8.226 |
Table S3 Thermodynamic parameters of Cr(III) adsorption on Q-SBA-15
| Sample | Tempera- ture/K | Kc | ΔG/ (kJ·mol-1) | ΔS/ (kJ·mol·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|---|---|
| Q-SBA-15 | 293 | 1.846 | -4.386 | 0.128 | 33.118 |
| 303 | 2.095 | -5.666 | |||
| 313 | 2.650 | -6.946 | |||
| 318 | 2.780 | -7.586 | |||
| 323 | 3.088 | -8.226 |
| [1] |
ALAGU K, VENU H, JAYARAMAN J, et al. Novel water hyacinth biodiesel as a potential alternative fuel for existing unmodified diesel engine: performance, combustion and emission characteristics. Energy, 2004, 179:295-305.
DOI URL |
| [2] | EBO D A, NANNE D V, BIRITWUM N K. Assessment of heavy metal pollution in the main Pra River and its tributaries in the Pra Basin of Ghana. Environmental Nanotechnology Monitoring & Management, 2018, 10:264-271. |
| [3] |
HASHEM M A, ISLAM A, MOHSIN S, et al. Green environment suffers by discharging of high-chromium-containing wastewater from the tanneries at Hazaribagh. Bangladesh. Sustainable Water Resources Management, 2015, 1(4):343-347.
DOI URL |
| [4] |
EL-SHAHAWI M S, HASSAN S M, OTHMAN A M, et al. Retention profile and subsequent chemical speciation of chromium(III) and (VI) in industrial wastewater samples employing some onium cations loaded polyurethane foams. Microchemical Journal, 2008, 89(1):13-19.
DOI URL |
| [5] |
BULUT V N, OZDES D, BEKIRCAN O, et al. Carrier element- free coprecipitation (CEFC) method for the separation, preconcentration and speciation of chromium using an isatin derivative. Analytica Chimica Acta, 2009, 632(1):35-41.
DOI URL |
| [6] |
MAO C P, SONG Y X, CHEN L X, et al. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena, 2019, 175:339-348.
DOI URL |
| [7] |
LIU X L, PANG H W, LIU X W, et al. Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. The Innovation, 2021, 2(1):100076.
DOI URL |
| [8] | WANG X X, LI X, WANG J Q, et al. Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution. Journal of Inorganic Materials, 2020, 35:260-270. |
| [9] |
GAO X P, GUO C, ZHAO Z, et al. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. International Journal of Biological Macromolecules, 2020, 164(1):4423-4434.
DOI URL |
| [10] |
CHIRANGANO M, TONNI A K, AHMAD B A. Comparative biosorption of chromium(VI) using chemically modified date pits (CM-DP) and olive stone (CM-OS): kinetics, isotherms and influence of co-existing ions. Chemical Engineering Research and Design, 2020, 156:251-262.
DOI URL |
| [11] |
GODIYA C B, CHENG X, LI D, et al. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. Journal of Hazardous Materials, 2018, 364(1):28-38.
DOI URL |
| [12] |
GIL C, MARIA L, FERRI A, et al. Distribution of chromium species in a Cr-polluted soil: presence of Cr(III) in glomalin related protein fraction. Science of the Total Environment, 2014, 493:828-833.
DOI URL |
| [13] |
ATIKAH M N, PEI S G, MOHD S A, et al. Adsorptive nanocomposite membranes for heavy metal remediation: recent progresses and challenges. Chemosphere, 2019, 232:96-112.
DOI URL |
| [14] |
RUIHUA M, BIN L, XI C, et al. Adsorption of Cu(II)and Co(II) from aqueous solution using lignosulfonate/chitosan adsorbent. International Journal of Biological Macromolecules, 2020, 163(1):120-127.
DOI URL |
| [15] |
BERA A, TRIVEDI J S, KUMAR S B, et al. Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions. Journal of Hazardous Materials, 2018, 343:86-97.
DOI URL |
| [16] |
WU H M, XIAO Y, GUO Y, et al. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ion. Microporous and Mesoporous Materials, 2020, 292:109754.
DOI URL |
| [17] |
CAROLIN C F, KUMAR P S, SARAVANAN A, et al. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. Journal of Environmental Chemical Engineering, 2017, 5(3):2782-2799.
DOI URL |
| [18] |
SURENDRAN P, ANEESH M, ANANDHU M, et al. Chelation dependent selective adsorption of metal ions by Schiff base modified SBA-15 from aqueous solutions. Journal of Environmental Chemical Engineering, 2020, 8(5):104248.
DOI URL |
| [19] | BETIHA M A, MOUSTAFA Y M, EL-SHAHAT M F, et al. Polyvinylpyrrolidone-aminopropyl-SBA-15 Schiff-base hybrid for efficient removal of divalent heavy metal cations from wastewater. Journal of Hazardous Materials, 2020, 397:112675. |
| [20] | XIAO Y, GUO Y, WU H M, et al. Adsorption of chromium(III) ions with amino functionalized mesoporous silica adsorbent. Chemical Industry and Engineering Progress, 2020, 30(9):263-272. |
| [21] |
CE L, WANG B D, HAN Y F, et al. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Microporous and Mesoporous Materials, 2017, 252:105-115.
DOI URL |
| [22] |
MOHAMMAD M S, ALI S R, MEHDI A, et al. Functionalization of SBA-15 by dithiooxamide towards removal of Co(II) ions from real samples: isotherm, thermodynamic and kinetic studies. Advanced Powder Technology, 2019, 30(9):1823-1834.
DOI URL |
| [23] |
ANBARASU G, MALATHY M, KARTHIKEYAN P, et al. Silica functionalized Cu(II) acetylacetonate Schiff base complex: an efficient catalyst for the oxidative condensation reaction of benzyl alcohol with amines. Journal of Solid State Chemistry, 2017, 253:305-312.
DOI URL |
| [24] |
HUANG S J, MA C Z, LIAO Y Z, et al. Superb adsorption capacity and mechanism of poly(1-amino-5-chloroanthraquinone) nanofibrils for lead and trivalent chromium ions. Reactive and Functional Polymers, 2016, 106:76-85.
DOI URL |
| [25] |
NICALAS F, FRANCISCO J, PEREZ A, et al. Chromium(VI) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents. Microporous and Mesoporous Materials, 2017, 239:138-146.
DOI URL |
| [26] |
KRISHNA S K, YADAV D, SHARMA S K, et al. Cu(II) Schiff base complex grafted guar gum: catalyst for benzophenone derivatives synthesis. Applied Catalysis A: General, 2020, 601:117529.
DOI URL |
| [27] |
HEMANDEZ-MORALES V, NAVA R, ACOSTA-SILVA Y J, et al. Adsorption of lead(II) on SBA-15 mesoporous molecular sieve functionalized with -NH2 groups. Microporous and Mesoporous Materials, 2012, 160:133-142.
DOI URL |
| [28] |
FELLENZ N, PEREZ-ALONSO F J, MARTIN P P, et al. Chromium(VI) removal from water by means of adsorption- reduction at the surface of amino-functionalized MCM-41 sorbents. Microporous and Mesoporous Materials, 2017, 239:138-146.
DOI URL |
| [29] |
CHEN F Y, HONG M Z, YOU W J, et al. Simultaneous efficient adsorption of Pb2+ and MnO4- ions by MCM-41 functionalized with amine and nitrilotriacetic acid anhydride. Applied Surface Science, 2015, 357:856-865.
DOI URL |
| [30] |
LIU S, CUI H Z, LI Y L, et al. Bis-pyrazolyl functionalized mesoporous SBA-15 for the extraction of Cr(III) and detection of Cr(VI) in artificial jewelry samples. Microchemical Journal, 2017, 131:130-136.
DOI URL |
| [31] |
KUMAR P A, RAY M, CHAKRABORTY S. Adsorption behaviour of trivalent chromium on amine-based polymer aniline formaldehyde condensate. Chemical Engineering Journal, 2009, 149(1/2/3):340-347.
DOI URL |
| [32] | WAN L, TONG S T. Adsorption of Cr3+ from aqueous solution on mesoporous activated carbon. Environmental Protection of Chemical industry, 2012, 32(1):75-80. |
| [33] | ZHANG X P. Modification of UIO-66-NH2 by 3, 4-dihydroxy benzaldehyde and its adsorption properties for U(VI). Rubber and Plastic Technology and Equipment, 2021, 47(2):43-50. |
| [34] | YI C L, YE X, LI T L, et al. Study the adsorption characteristics of biomanganese oxide to four heavy metals. Industrial Safety and Environmental Protection, 2021, 47(3):94-98. |
| [35] | CHENG FU-QIANG, JI TIAN-TIAN, XUE MIN, et al. Preparation of thiohydroxy-functionalized mesoporous materials and its adsorption to Cr6+. Journal of Inorganic Materials, 2020, 35(2):194-198. |
| [36] | ZHU MING-YU, FAN DE-ZE, LIU BEI, et al. C@K2Ti6O13 hierarchical nano materials: effective adsorption removel of Cr(VI). Journal of Inorganic Materials, 2020, 35(3):310-314. |
| [37] | PONCE-LIRA B, OTAZO-SÁNCHEZ E M, REGUERA E, et al. Lead removal from aqueous solution by basaltic scoria: adsorption equilibrium and kinetics. International Journal of Environmental Science and Technology, 2017, 14:1181-1196. |
| [38] | SONG X T, NIU Y Z, ZHANG P P, et al. Removal of Co(II) from fuel ethanol by silica-gel supported PAMAM dendrimers: combined experimental and theoretical study. Fuel, 2017, 199(1):91-101. |
| [39] | BHAUMIK M, MAITY A, SRINIVASU V V, et al. Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. Journal of Hazardous Materials, 2011, 190(1/2/3):381-390. |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [3] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [4] | WU Guangyu, SHU Song, ZHANG Hongwei, LI Jianjun. Enhanced Styrene Adsorption by Grafted Lactone-based Activated Carbon [J]. Journal of Inorganic Materials, 2024, 39(4): 390-398. |
| [5] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| [6] | CHAO Shaofei, XUE Yanhui, WU Qiong, WU Fufa, MUHAMMAD Sufyan Javed, ZHANG Wei. Efficient Potassium Storage through Ti-O-H-O Electron Fast Track of MXene Heterojunction [J]. Journal of Inorganic Materials, 2024, 39(11): 1212-1220. |
| [7] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [8] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [9] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [10] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [11] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [12] | TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. |
| [13] | DAI Jieyan, FENG Aihu, MI Le, YU Yang, CUI Yuanyuan, YU Yun. Adsorption Mechanism of NaY Zeolite Molecular Adsorber Coating on Typical Space Contaminations [J]. Journal of Inorganic Materials, 2023, 38(10): 1237-1244. |
| [14] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| [15] | LIU Cheng, ZHAO Qian, MOU Zhiwei, LEI Jiehong, DUAN Tao. Adsorption Properties of Novel Bismuth-based SiOCNF Composite Membrane for Radioactive Gaseous Iodine [J]. Journal of Inorganic Materials, 2022, 37(10): 1043-1050. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||