
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (6): 697-702.DOI: 10.15541/jim20190290
• RESEARCH PAPER • Previous Articles Next Articles
ZHAI Wanru1,WANG Jiahui1,WANG Maohuai1,DU Xuemei1,WEI Shuxian2( )
)
Received:2019-06-13
Revised:2019-07-08
Published:2020-06-20
Online:2019-07-23
Supported by:CLC Number:
ZHAI Wanru,WANG Jiahui,WANG Maohuai,DU Xuemei,WEI Shuxian. Adsorption and Separation of CO2/N2 in Metal Organic Frameworks: a Theoretical Investigation[J]. Journal of Inorganic Materials, 2020, 35(6): 697-702.
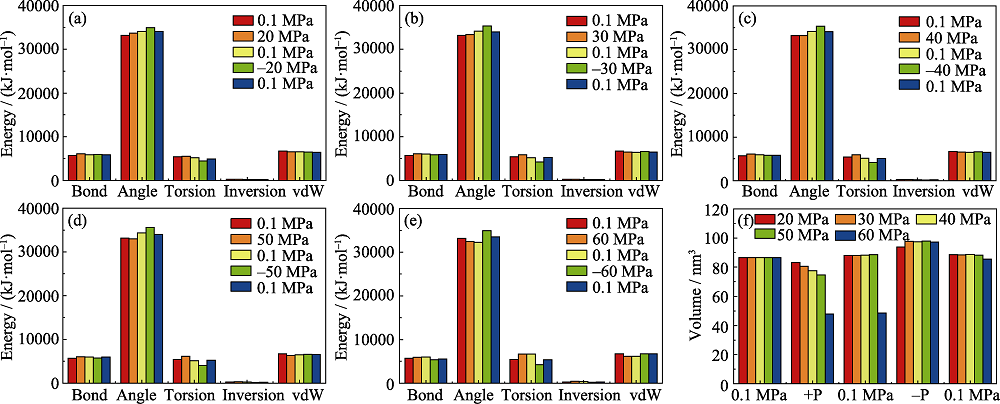
Fig. 3 Energy changes at (a) 20, (b) 30, (c) 40, (d) 50 and (e) 60 MPa and (f) volume changes at 20-60 MPa in the structure transformation process of DUT-49
| [1] |
DANG Q Q, LIU C Y, WANG X M , et al. Novel covalent triazine framework for high-performance CO2 capture and alkyne carboxylation reaction. ACS Applied Materials & Interfaces, 2018,10(33):27972-27978.
DOI URL |
| [2] |
TORSÆTER M, CERASI P . Geological and geomechanical factors impacting loss of near-well permeability during CO2 injection. International Journal of Greenhouse Gas Control, 2018,76:193-199.
DOI URL |
| [3] |
KARL T R, TRENBERTH K E . Modern global climate change. Science, 2003,302(5651):1719-1723.
DOI URL |
| [4] |
LIN Y C, KONG C L, ZHANG Q J , et al. Metal-organic frameworks for carbon dioxide capture and methane storage. Advanced Energy Materials, 2017,7(4):1601296.
DOI URL |
| [5] |
LU X Q, JIN D L, WEI S X , et al. Strategies to enhance CO2 capture and separation based on engineering absorbent materials. Journal of Materials Chemistry A, 2015,3(23):12118-12132.
DOI URL |
| [6] |
WANG M H, WEI S X, WU Z H , et al. Alkyl amine functionalized triphenylamine-based covalent organic frameworks for high- efficiency CO2 capture and separation over N2. Materials Letters, 2018,230:28-31.
DOI URL |
| [7] |
SUMIDA K, ROGOW D L, MASON J A , et al. Carbon dioxide capture in metal-organic frameworks. Chemical Reviews, 2011,112(2):724-781.
DOI URL |
| [8] |
SEOANE B, CaSTELLANOS S, DiKHTIARENKO A, et al. Multi- scale crystal engineering of metal organic frameworks. Coordination Chemistry Reviews, 2016,307:147-187.
DOI URL |
| [9] | XIANG Z H, CAO D P, LAN J H , et al. Multiscale simulation and modelling of adsorptive processes for energy gas storage and carbon dioxide capture in porous coordination frameworks. Energy & Environmental Science, 2010,3(10):1469-1487. |
| [10] |
MILLWARD A R, YAGHI O M . Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. Journal of the American Chemical Society, 2005,127(51):17998-17999.
DOI URL |
| [11] |
DEMESSENCE A, D’ALESSANDRO D M, FOO M L, et al. Strong CO2 binding in a water-stable, triazolate-bridged metal-organic framework functionalized with ethylenediamine. Journal of the American Chemical Society, 2009,131(25):8784-8786.
DOI URL |
| [12] |
LIU Y, WANG Z U, ZHOU H C . Recent advances in carbon dioxide capture with metal-organic frameworks. Greenhouse Gases: Science and Technology, 2012,2(4):239-259.
DOI URL |
| [13] |
ZHANG Z J, ZHAO Y G, GONG Q H , et al. MOFs for CO2 capture and separation from flue gas mixtures: the effect of multifunctional sites on their adsorption capacity and selectivity. Chemical Communications, 2013,49(7):653-661.
DOI URL |
| [14] |
KRAUSE S, BON V, SENKOVSKA I , et al. A pressure-amplifying framework material with negative gas adsorption transitions. Nature, 2016,532(7599):348.
DOI URL |
| [15] |
EVANS J D, BOCQUET L, COUDERT F . X. Origins of negative gas adsorption. Chem, 2016,1(6):873-886.
DOI URL |
| [16] |
POTOFF J J, SIEPMANN J I . Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE Journal, 2001,47(7):1676-1682.
DOI URL |
| [17] |
RAPPÉ A K, CASEWIT C J, COLWELL K , et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. Journal of the American Chemical Society, 1992,114(25):10024-10035.
DOI URL |
| [18] |
GUPTA A, CHEMPATH S, SANBORN M J , et al. Object-oriented programming paradigms for molecular modeling. Molecular Simulation, 2003,29(1):29-46.
DOI URL |
| [19] | LIU J Y, LIU S . A survey on applications of Voronoi diagrams. Journal of Engineering Graphics, 2004,2:125-132. |
| [20] |
AHMED A, BABARAO R, HUANG R , et al. Porous aromatic frameworks impregnated with lithiated fullerenes for natural gas purification. The Journal of Physical Chemistry C, 2015,119(17):9347-9354.
DOI URL |
| [1] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [2] | HONG Jiahui, MA Ran, WU Yunchao, WEN Tao, AI Yuejie. CoNx/g-C3N4 Nanomaterials Preparation by MOFs Self-sacrificing Template Method for Efficient Photocatalytic Reduction of U(VI) [J]. Journal of Inorganic Materials, 2022, 37(7): 741-749. |
| [3] | WANG Jing, XU Shoudong, LU Zhonghua, ZHAO Zhuangzhuang, CHEN Liang, ZHANG Ding, GUO Chunli. Hollow-structured CoSe2/C Anode Materials: Preparation and Sodium Storage Properties for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(12): 1344-1350. |
| [4] | LI Guodong, JI Guoxun, SUN Xinli, DU Wei, LIU Wei, WANG Shuao. Layered Metal Organic Framework for Effective Removal of 137Cs from Aqueous Solution [J]. Journal of Inorganic Materials, 2020, 35(3): 367-372. |
| [5] | ZHANG Yi-Qing, LIU Li, ZHANG Shu-Juan, WAN Zheng-Rui, LIU Hong-Ying, ZHOU Li-Qun. Preparation and Dehydrogenation Property of NH2-UIO-66 Supported RuCuMo Nanocatalyst [J]. Journal of Inorganic Materials, 2019, 34(12): 1316-1324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||