
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (4): 387-394.DOI: 10.15541/jim20210354
• RESEARCH ARTICLE • Previous Articles Next Articles
DONG Shurui( ), ZHAO Di, ZHAO Jing(
), ZHAO Di, ZHAO Jing( ), JIN Wanqin
), JIN Wanqin
Received:2021-06-04
Revised:2021-08-17
Published:2022-04-20
Online:2021-08-20
Contact:
ZHAO Jing, associate professor. E-mail: zhaojingmem@njtech.edu.cnAbout author:DONG Shurui (1988-), female, Master candidate. E-mail: dsr@njtech.edu.cn
Supported by:CLC Number:
DONG Shurui, ZHAO Di, ZHAO Jing, JIN Wanqin. Effect of Ionized Amino Acid on the Water-selective Permeation through Graphene Oxide Membrane in Pervaporation Process[J]. Journal of Inorganic Materials, 2022, 37(4): 387-394.
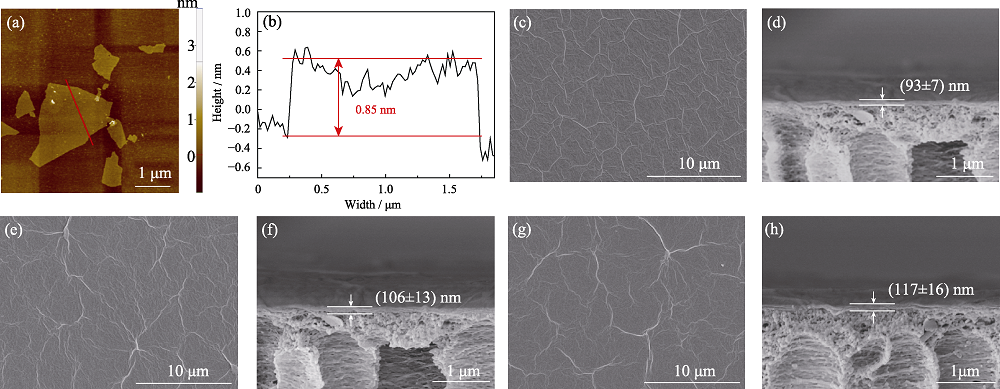
Fig. 2 (a) AFM image and (b) height profile of GO nanosheet, and FESEM images of the surface and cross-section of (c, d) pristine GO, (e, f) Lys(10)-GO, and (g, h) Lys(Na+)(10)-GO membranes
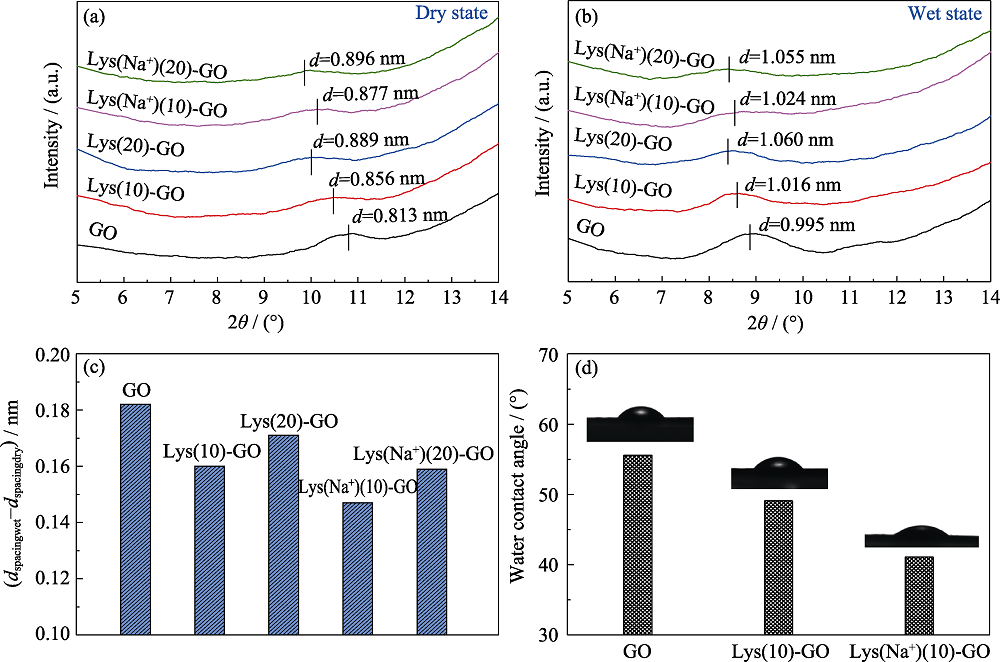
Fig. 4 XRD patterns of GO, Lys(10)-GO and Lys(Na+)(10)-GO membranes in (a) dry state and (b) wet state, (c) differences in the interlayer spacing of different films in dry and wet states and (d) water contact angle of pristine GO, Lys(10)-GO and Lys(Na+)(10)-GO membranes
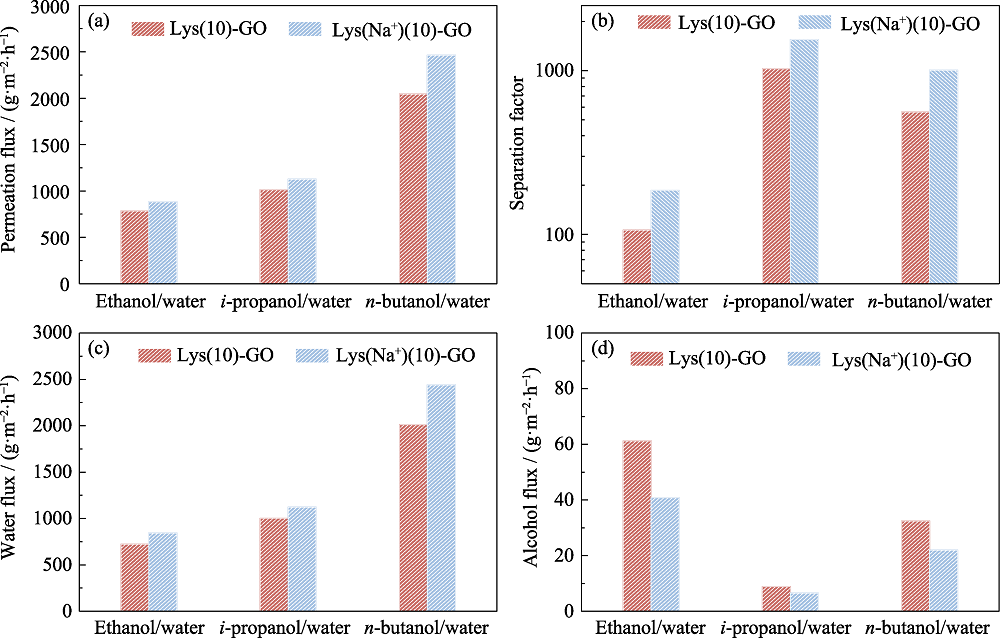
Fig. 6 Separation performance of Lys(10)-GO and Lys(Na+)(10)-GO membranes for separating different water/alcohol mixtures (a) Permeation flux; (b) Separation factor; (c) Water flux; (d) Alcohol flux
| Membrane | Feed solution | Temperature/℃ | Permeation flux/(g·m-2·h-1) | Separation factor | PSI (×105) | Reference |
|---|---|---|---|---|---|---|
| Lys(Na+)(10)-GO | 90% n-butanol | 40 | 2461 | 1005 | 24.7 | This work |
| 1%IL-GO-PEBA | 98% n-butanol | 35 | 478.3 | 26.7 | 0.12 | [ |
| GO-PMDTT | 85% n-butanol | 40 | 973 | 99 | 0.95 | [ |
| PDMS-PhTMS/PVDF | 85% n-butanol | 60 | 850 | 1174 | 9.97 | [ |
| ZIF-8@PPy30/PDMS | 95% n-butanol | 40 | 564.8 | 70.2 | 0.39 | [ |
| Lys(Na+)-GO | 90% i-propanol | 40 | 1127 | 1543 | 17.4 | This work |
| GO-GTA | 85% i-propanol | 40 | 700 | 1800 | 12.6 | [ |
| PC-0 | 88% i-propanol | 40 | 844 | 711 | 5.99 | [ |
| PVA-b-NaY | 90% i-propanol | 35 | 5.12 | 2690 | 0.14 | [ |
| Lys(Na+)-GO | 90% ethanol | 40 | 882 | 186 | 1.63 | This work |
| ZIF-8@GO | 95% ethanol | 40 | 443.8 | 22.2 | 0.09 | [ |
| PVA- GO | 90% ethanol | 40 | 137 | 263 | 0.36 | [ |
| PEGDA-GO | 90% ethanol | 40 | 700 | 70 | 0.48 | [ |
| CaGO | 90% ethanol | 40 | 430 | 141 | 0.6 | [ |
| GO/ceramic | 90% ethanol | 40 | 430 | 335 | 1.43 | [ |
| AZIF-8@PDMS-7 MMM | 95% ethanol | 40 | 585.6 | 17.7 | 0.10 | [ |
Table 1 Summary of the performance of membranes for pervaporation dehydration of water/alcohol mixtures
| Membrane | Feed solution | Temperature/℃ | Permeation flux/(g·m-2·h-1) | Separation factor | PSI (×105) | Reference |
|---|---|---|---|---|---|---|
| Lys(Na+)(10)-GO | 90% n-butanol | 40 | 2461 | 1005 | 24.7 | This work |
| 1%IL-GO-PEBA | 98% n-butanol | 35 | 478.3 | 26.7 | 0.12 | [ |
| GO-PMDTT | 85% n-butanol | 40 | 973 | 99 | 0.95 | [ |
| PDMS-PhTMS/PVDF | 85% n-butanol | 60 | 850 | 1174 | 9.97 | [ |
| ZIF-8@PPy30/PDMS | 95% n-butanol | 40 | 564.8 | 70.2 | 0.39 | [ |
| Lys(Na+)-GO | 90% i-propanol | 40 | 1127 | 1543 | 17.4 | This work |
| GO-GTA | 85% i-propanol | 40 | 700 | 1800 | 12.6 | [ |
| PC-0 | 88% i-propanol | 40 | 844 | 711 | 5.99 | [ |
| PVA-b-NaY | 90% i-propanol | 35 | 5.12 | 2690 | 0.14 | [ |
| Lys(Na+)-GO | 90% ethanol | 40 | 882 | 186 | 1.63 | This work |
| ZIF-8@GO | 95% ethanol | 40 | 443.8 | 22.2 | 0.09 | [ |
| PVA- GO | 90% ethanol | 40 | 137 | 263 | 0.36 | [ |
| PEGDA-GO | 90% ethanol | 40 | 700 | 70 | 0.48 | [ |
| CaGO | 90% ethanol | 40 | 430 | 141 | 0.6 | [ |
| GO/ceramic | 90% ethanol | 40 | 430 | 335 | 1.43 | [ |
| AZIF-8@PDMS-7 MMM | 95% ethanol | 40 | 585.6 | 17.7 | 0.10 | [ |
| [1] |
ZHAO J, HE G W, LIU G H, et al. Manipulation of interactions at membrane interfaces for energy and environmental applications. Progress in Polymer Science, 2018, 80: 125-152.
DOI URL |
| [2] |
HUA D, CHUNG T S, SHI G M, FANG C. Teflon AF2400/Ultem composite hollow fiber membranes for alcohol dehydration by high-temperature vapor permeation. AIChE Journal, 2016, 62(5): 1747-1757.
DOI URL |
| [3] |
PRIHATININGTYAS I, BRUGGEN B V D. Nanocomposite pervaporation membrane for desalination. Chemical Engineering Research and Design, 2020, 164: 147-161.
DOI URL |
| [4] | LIANG F, LIU Q, ZHAO J, et al. Ultrafast water-selective permeation through graphene oxide membrane with water transport promoters. AIChE Journal, 2020, 66(2):e16812. |
| [5] |
DAI L, XU F, HUANG K, et al. Ultrafast water transport in two-dimensional channels enabled by spherical polyelectrolyte brushes with controllable flexibility. Angew. Chem. Int. Ed., 2021, 60(36): 19933-19941.
DOI URL |
| [6] |
NAIR R R, WU H A, JAYARAM P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science, 2012, 335(6067): 442-444.
DOI URL |
| [7] |
WANG M, PAN F, YANG L, et al. Graphene oxide quantum dots incorporated nanocomposite membranes with high water flux for pervaporative dehydration. Journal of Membrane Science, 2018, 563: 903-913.
DOI URL |
| [8] |
SONG Y, LI R, PAN F, et al. Ultrapermeable graphene oxide membranes with tunable interlayer distances via vein-likesupramolecular dendrimers. J. Mater. Chem. A, 2019, 7(31): 18642-18652.
DOI URL |
| [9] |
DREYER D R, PARK S, BIELAWSKI C W, et al. The chemistry of graphene oxide. Chemical Society Reviews, 2010, 39(1): 228-240.
DOI URL |
| [10] |
LIU L, MA Q, CAO J, et al. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. Cancer Nano, 2021, 12: 18.
DOI URL |
| [11] |
SUN P, ZHU M, WANG K, et al. Selective ion penetration of graphene oxide membranes. ACS Nano, 2013, 7(1): 428-437.
DOI URL |
| [12] |
CHOWDHURY I, MANSUKHANI N D, GUINEY L M, et al. Aggregation and stability of reduced graphene oxide: complex roles of divalent cations, pH, and natural organic matter. Environmental Science & Technology, 2015, 49(18): 10886-10893.
DOI URL |
| [13] |
SUN P, ZHENG F, ZHU M, et al. Selective trans-membrane transport of alkali and alkaline earth cations through graphene oxide membranes based on cation-π interactions. ACS Nano, 2014, 8(1): 850-859.
DOI URL |
| [14] |
TANG W, LOU H, LI Y, et al. Ionic liquid modified graphene oxide-PEBA mixed matrix membrane for pervaporation of butanol aqueous solutions. Journal of Membrane Science, 2019, 581: 93-104.
DOI URL |
| [15] |
MANSHAD S, ISLOOR A M, NAWAWI M G M, et al. Pervaporation dehydration of bio-fuel (n-butanol) by dry thermal treatment membrane. Materials Research Express, 2020, 7(6):065001.
DOI URL |
| [16] |
LEE J Y, LEE J S, LEE J. P High performance and thermally stable PDMS pervaporation membranes prepared using a phenyl-containing tri-functional crosslinker for n-butanol recovery. Separation and Purification Technology, 2020, 235: 116142.
DOI URL |
| [17] |
XU L, LI S, MAO H, et al. An advanced necklace-like metal organic framework with an ultrahighly continuous structure in the membrane for superior butanol/water separation. J. Mater. Chem. A, 2021, 9(19):11853.
DOI URL |
| [18] |
HUA D, RAI R K, ZHANG Y, et al. Aldehyde functionalized graphene oxide frameworks as robust membrane materials for pervaporative alcohol dehydration. Chemical Engineering Science, 2017, 161: 341-349.
DOI URL |
| [19] |
ACHRI D, RACHIPUDI P, NAIK S, et al. Polyelectrolyte complex membranes made of chitosan-PSSAMA for pervaporation separation of industrially important azeotropic mixtures. Journal of Industrial and Engineering Chemistry, 2019, 78: 383-395.
DOI URL |
| [20] |
KURSUN F. Application of PVA-b-NaY zeolite mixture membranes in pervaporation method. Journal of Molecular Structure, 2020, 1201: 127170.
DOI URL |
| [21] |
ZHU T, XU S, YU F, et al. ZIF-8@GO composites incorporated polydimethylsiloxane membrane with prominent separation performance for ethanol recovery. Journal of Membrane Science, 2020, 598: 117681.
DOI URL |
| [22] |
ZHAO D, JI Y F, LIU G P, et al. Facilitated water-selective permeation via PEGylation of graphene oxide membrane. Journal of Membrane Science, 2018, 567: 311-320
DOI URL |
| [23] |
CASTRO-MUNOZ R, BUERA-GONZALEZ J, IGLESIA, et al. Towards the dehydration of ethanol using pervaporation cross- linked poly(vinyl alcohol)/graphene oxide membranes. Journal of Membrane Science, 2019, 582: 423-484.
DOI URL |
| [24] |
GUAN K, LIU Q, ZHOU G, et al. Cation-diffusion controlled formation of thin graphene oxide composite membranes for efficient ethanol dehydration. Science China Materials, 2019, 62(7): 925-935.
DOI URL |
| [25] |
CHENG X, CAI W, CHEN X, et al. Preparation of graphene oxide/poly(vinyl alcohol) composite membrane and pervaporation performance for ethanol dehydration. RSC Advances, 2019, 9(27): 15457-15465
DOI URL |
| [26] |
ZHU T, LE YU X, YI M, et al. Facile covalent crosslinking of zeolitic imidazolate framework/polydimethylsiloxane mixed matrix membrane for enhanced ethanol/water separation performance. ACS Sustainable Chem. Eng., 2020, 8(33): 12664-12676.
DOI URL |
| [1] | ZHANG Bo,ZHANG Ning,YANG Jianhua,LAN Jiancheng,WANG Jinqu. High Performance a&b Oriented T Zeolite Membrane by a Two-stage Crystallization Synthesis [J]. Journal of Inorganic Materials, 2020, 35(8): 939-946. |
| [2] | ZHU Chunhui, XU Rong, REN Xiuxiu, ZUO Shixiang, GONG Genghao, ZHONG Jing. Fabrication of ZIF-8-NH2/Organosilica Hybrid Membranes for Pervaporation Desalination [J]. Journal of Inorganic Materials, 2020, 35(11): 1239-1246. |
| [3] | HUANG Pan, ZHOU Liang, LI Hua-Zheng, LI Hong-Jian, YANG Jian-hua, WANG Jin-Qu. Preparation and Characterization of High Performance MFI Zeolite Membrane in Ultradilute Solution [J]. Journal of Inorganic Materials, 2018, 33(3): 345-351. |
| [4] | WANG Xiao-Lei, ZHANG Yu-Ting, GAO Bing, ZHANG Chun, GU Xue-Hong. Preparation and Characterization of NaA Zeolite Membranes on Inner-surface of Four-channel Ceramic Hollow Fibers [J]. Journal of Inorganic Materials, 2018, 33(3): 339-344. |
| [5] | LV You-Jia, LI Hua-Zheng, YANG Jian-Hua, WANG Jin-Qu, YIN De-Hong, LU Jin-Ming. Preparation and Characterization of High Performance Zeolite T Membranes from Clear Solutions [J]. Journal of Inorganic Materials, 2016, 31(7): 705-710. |
| [6] | ZHOU Liang, YANG Jian-Hua, WANG Jin-Qu, LU Jin-Ming, ZHANG Yan, YIN De-Hong. Synthesis of SAPO-34 Molecular Sieve Membranes by Steam-assisted Conversion Seeding and Their Characterization [J]. Journal of Inorganic Materials, 2015, 30(3): 294-298. |
| [7] | LI Liang-Qing, ZHANG Wen-Xu, YANG Jian-Hua, LU Jin-Ming, YIN De-Hong, WANG Jin-Qu. Preparation and Characterization of Water Perm-selectivity ZSM-5 Zeolite Membrane Using Fluoride Route [J]. Journal of Inorganic Materials, 2015, 30(11): 1167-1171. |
| [8] | WANG Xian-Wu,YANG Jian-Hua,LU Jin-Ming,WANG Tong-Hua,WANG Jin-Qu. Synthesis of Sn-Substituted ZSM-5/Carbon Zeolite Membrane and Application in Separation of Acetic Acid/water Mixtures by Pervaporation [J]. Journal of Inorganic Materials, 2008, 23(6): 1225-1230. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||