
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (4): 395-403.DOI: 10.15541/jim20210255
• RESEARCH ARTICLE • Previous Articles Next Articles
MA Lei1,2( ), HUANG Yi1(
), HUANG Yi1( ), DENG Hao2, YIN Hang1,2, TIAN Qiang1, YAN Minghao1
), DENG Hao2, YIN Hang1,2, TIAN Qiang1, YAN Minghao1
Received:2021-04-15
Revised:2021-05-27
Published:2022-04-20
Online:2021-06-30
Contact:
HUANG Yi, lecturer. E-mail: huangyi516@163.comAbout author:MA Lei(1996-), male, Master candidate. E-mail: 2219835537@qq.com
Supported by:CLC Number:
MA Lei, HUANG Yi, DENG Hao, YIN Hang, TIAN Qiang, YAN Minghao. Removal of Uranium (VI) from Acidic Aqueous Solution by Fluorapatite[J]. Journal of Inorganic Materials, 2022, 37(4): 395-403.

Fig. 2 (a) Distribution of uranium (VI) species in the solution with different pH (C0=100 mg/L, T= 308 K); (b) Adsorption capacity and removal rate of uranium (VI) by fluorapatite in the solution with different pH; (c) Zeta potential of fluorapatite in the solution with different pH; (d) Effect of solid-liquid ratio on adsorption of uranium (VI) by fluorapatite (pH3); (e) Change of the adsorption of uranium (VI) by fluorapatite with adsorption time (pH3, solid-liquid ratio at 0.12 g/L); (f) Effect of initial uranium (VI) concentration on adsorption of uranium (VI) by fluorapatite (pH3, solid-liquid ratio at 0.12 g/L, adsorption time=120 min)
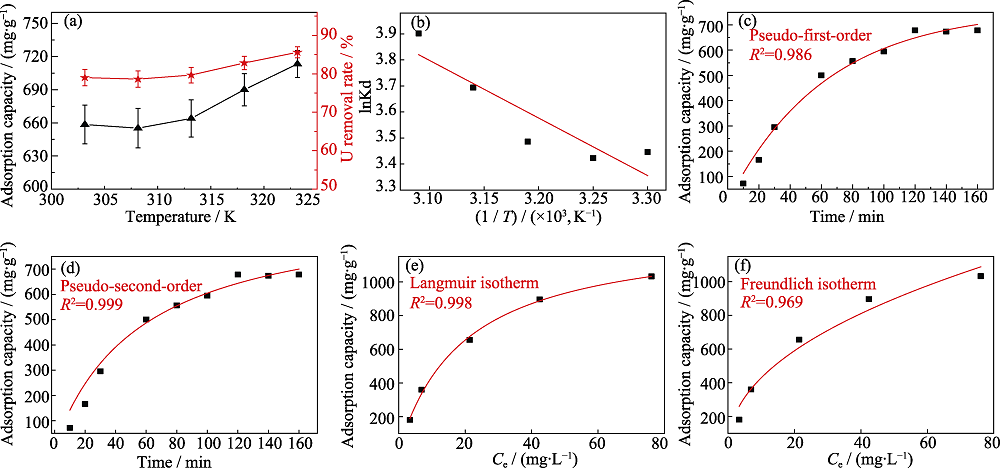
Fig. 4 (a) Adsorption capacity and removal rate of uranium (VI) by fluorapatite in the temperature range of 303-323 K; (b) Linear fitting of thermodynamic model for adsorption process; (c) Nonlinear fitting of pesudo-first-order kinetic model; (d) Nonlinear fitting of pesudo-second-order kinetic model; (e) Nonlinear fitting of Langmuir isotherm adsorption model; (f) Nonlinear fitting of Freundlich isotherm adsorption model
| Materials | ∆H/(kJ∙mol-1) | ∆S/(J∙mol-1•K-1) | ∆G/(kJ•mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Fluorapatite | 8.41 | 88.65 | -8.47 | -8.91 | -9.31 | -9.8 | -10.24 |
Table 1 Fitting parameters of thermodynamic model for adsorption
| Materials | ∆H/(kJ∙mol-1) | ∆S/(J∙mol-1•K-1) | ∆G/(kJ•mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Fluorapatite | 8.41 | 88.65 | -8.47 | -8.91 | -9.31 | -9.8 | -10.24 |
| Materials | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| K1/min-1 | R2 | K2 / (g∙mg-1∙min-1) | R2 | |
| Fluorapatite | 0.016 | 0.986 | 1.85×10-5 | 0.999 |
Table 2 Fitting parameters of pesudo-first-order and pesudo-second-order kinetic models
| Materials | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| K1/min-1 | R2 | K2 / (g∙mg-1∙min-1) | R2 | |
| Fluorapatite | 0.016 | 0.986 | 1.85×10-5 | 0.999 |
| Materials | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| qmax /(mg∙g-1) | R2 | n | R2 | ||
| Fluorapatite | 1300.35 | 0.998 | 2.18 | 0.969 | |
Table 3 Fitting parameters of Langmuir and Freundlich isothermal adsorption models
| Materials | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| qmax /(mg∙g-1) | R2 | n | R2 | ||
| Fluorapatite | 1300.35 | 0.998 | 2.18 | 0.969 | |
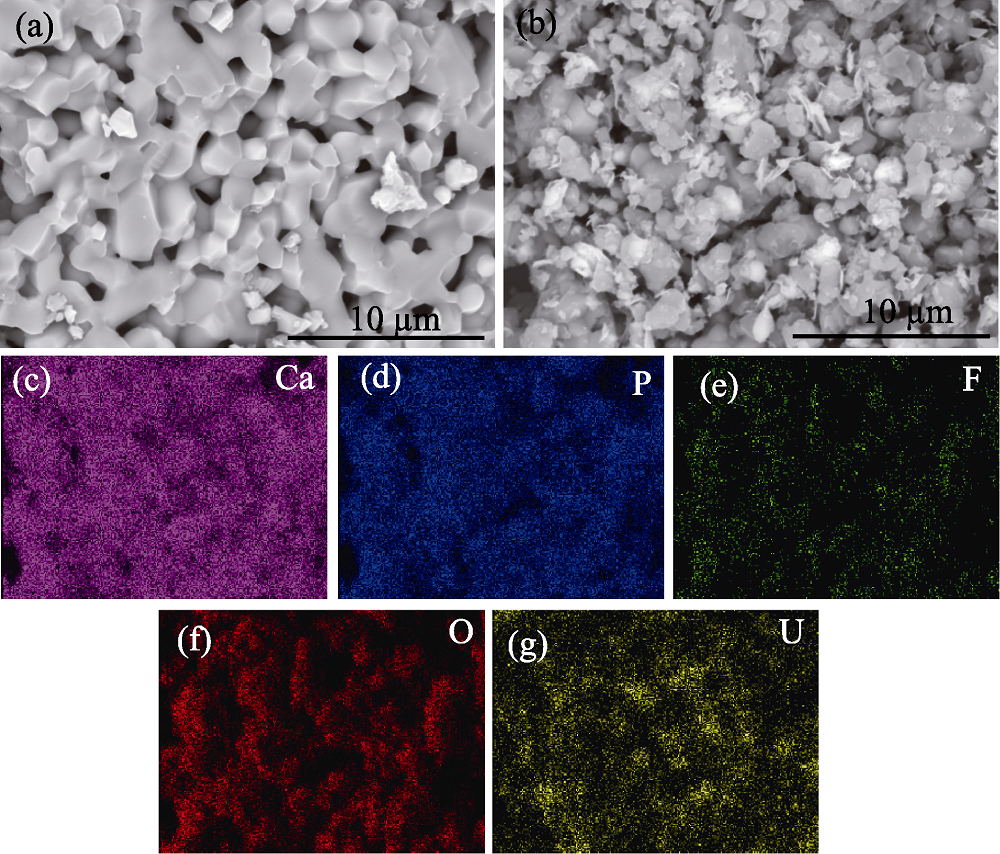
Fig. 5 SEM images of fluorapatite before (a) and after (b) adsorption of uranium (VI), and element mappings (c-g) of the surface of fluorapatite after adsorption of uranium (VI)
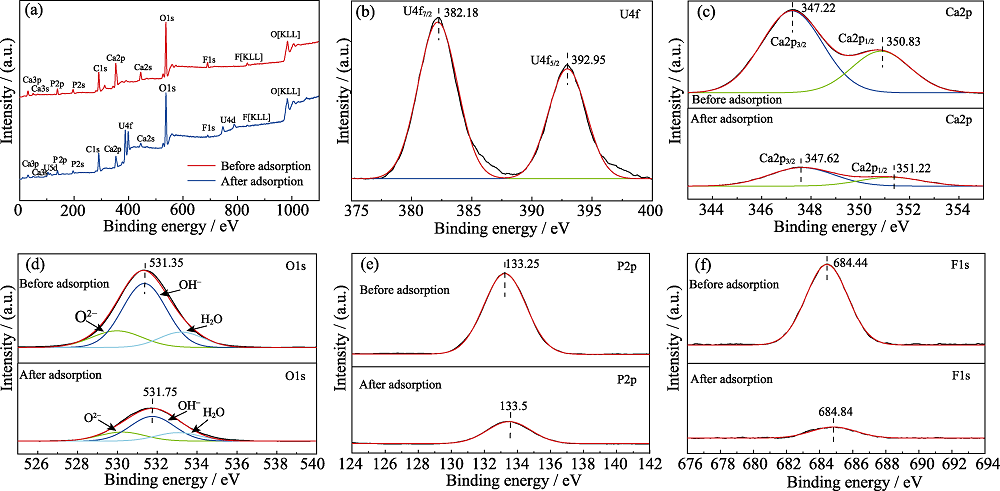
Fig. 6 (a) XPS spectra of fluorapatite before and after adsorption of uranium (VI) and (b) U4f on the fluorapatite after adsorption of uranium (VI) (b), and XPS spectra of Ca2p(c), O1s(d), P2p(e) and F1s(f) on the fluorapatite before and after adsorption of uranium (VI)
| [1] |
GIANNAKOUDAKIS D A, ANASTOPOULOS I, BARCZAK M, et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: surface chemistry matters the most. Journal of Hazardous Materials, 2021, 413: 125279.
DOI URL |
| [2] | WANG J Q, PANG H W, TANG H, et al. Study on the removal of U(VI) in water by carbon supported zero valent iron prepared by carbothermal reduction method. Journal of Inorganic Materials, 2020, 35(3): 373-380. |
| [3] | ZHANG Z B, ZHOU R Z, DONG Z M, et al. Effect of amidoxime hydrothermal carbon on U(VI)-CO3/Ca- U(VI)-CO3. Journal of Inorganic Materials, 2020, 35(03): 352-358. |
| [4] |
SALAMA E, EL-KAMEESY S U, ELRAWI R. Depleted uranium assessment and natural radioactivity monitoring in north west of Iraq over a decade since the last gulf war. Journal of Environmental Radioactivity, 2019, 201: 25-31.
DOI URL |
| [5] |
VENU-BABU P, SUSAN E. High efficiency phytoextraction of uranium using vetiveria zizanioides L. Nash. International Journal of Phytoremediation, 2020, 22(11): 1137-1146.
DOI URL |
| [6] |
YANG S Y, LI Q, CHEN L, et al. Synergistic removal and reduction of U(VI) and Cr(VI) by Fe3S4 micro-crystal. Chemical Engineering Journal, 2020, 385: 123909.
DOI URL |
| [7] |
WANG X L, THOMAS M J, CRAIG C L. Low temperature equilibrium isotope fractionation and isotope exchange kinetics between U(IV) and U(VI). Geochimica et Cosmochimica Acta, 2015, 158: 262-275.
DOI URL |
| [8] |
SUDESHNA S, HIRAKENDU B, ROUT S. et al. Nanohydroxyapatite coated activated carbon impregnated alginate: a new hybrid sorbent for uranium removal from potable water. Journal of Environmental Chemical Engineering, 2020, 8(4):103999.
DOI URL |
| [9] |
YU S J, WANG X X, LIU Y F, et al. Efficient removal of uranium(VI) by layered double hydroxides supported nanoscale zero-valent iron: a combined experimental and spectroscopic studies. Chemical Engineering Journal, 2019, 365: 51-59.
DOI URL |
| [10] |
DAI Y M, ZHOU L M, TANG X H, et al. Macroporous ion-imprinted chitosan foams for the selective biosorption of U(VI) from aqueous solution. International Journal of Biological Macromolecules, 2020, 164: 4155-4164.
DOI URL |
| [11] |
MA D H, WEI J J, ZHAO Y S, et al. The removal of uranium using novel temperature sensitive urea-formaldehyde resin: adsorption and fast regeneration. Science of the Total Environment, 2020, 735: 139399.
DOI URL |
| [12] |
LU W, DAI Z R, LI L, et al. Preparation of composite hydrogel (PCG)and its adsorption performance for uranium(VI). Journal of Molecular Liquids, 2020, 303: 112604.
DOI URL |
| [13] |
WU Y H, CHEN D Y, KONG L J, et al. Rapid and effective removal of uranium(VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. Journal of Hazardous Materials, 2019, 371: 397-405.
DOI URL |
| [14] |
SHI Q P, SU M H, YUVARAJA G, et al. Development of highly efficient bundle-like hydroxyapatite towards abatement of aqueous U(VI) ions: mechanism and economic assessment. Journal of Hazardous Materials, 2020, 394: 122550.
DOI URL |
| [15] |
HAN R P, ZOU W H, WANG Y, et al. Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. Journal of Environmental Radioactivity, 2007, 93(3): 127-143.
DOI URL |
| [16] |
CAMACHO L M, DENG S G, PARRA R R. Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. Journal of Hazardous Materials, 2010, 175(1/2/3): 393-398.
DOI URL |
| [17] |
ZONG P F, WU X Y, GOU J Y, et al. Immobilization and recovery of uranium (VI) using Na-bentonite from aqueous medium: equilibrium, kinetics and thermodynamics studies. Journal of Molecular Liquids, 2015, 209: 358-366.
DOI URL |
| [18] |
HOUHOUNE F, NIBOU D, CHEGROUCHE S, et al. Behaviour of modified hexadecyltrimethylammonium bromide bentonite toward uranium species. Journal of Environmental Chemical Engineering, 2016, 4(3): 3459-3467.
DOI URL |
| [19] |
MARIA E, IOANNIS P. A comparative study of the adsorption of uranium on commercial and natural (Cypriot) sea sand samples. Journal of Radioanalytical and Nuclear Chemistry, 2013, 298(2): 1111-1116.
DOI URL |
| [20] |
HU W, ZHANG Z X, LI M X, et al. Enhanced uptake capacity for uranium (VI) in aqueous solutions by activated natural siderite: performance and mechanism. Applied Geochemistry, 2019, 100: 96-103.
DOI URL |
| [21] |
BENGTSSON A, SHCHUKAREV A, PERSSON P, et al. Phase transformations, ion-exchange, adsorption, and dissolution processes in aquatic fluorapatite systems. Langmuir, 2009, 25(4): 2355-2362.
DOI URL |
| [22] | VALYASHKO V M, KOGARKO L N, KHODAKOVSKIY I L. Stability of fluorapatite, chlorapatite and hydroxylapatite in aqueous solutions at different temperatures. Geochemistry International, 1968, 5(1): 21-30. |
| [23] |
HUANG Y, ZHANG H B, ZHOU X S, et al. Synthesis and microstructure of fluorapatite-type Ca10-2xSmxNax(PO4)6F2 solid solutions for immobilization of trivalent minor actinide. Journal of Nuclear Materials, 2017, 485: 105-112.
DOI URL |
| [24] |
BROS R, CARPENA J, SERE V, et al. Occurrence of Pu and fissiogenic REE in hydrothermal apatites from the fossil nuclear reactor 16 at Oklo (Gabon). Radiochimica Acta, 2013, 74(s1): 277-282.
DOI URL |
| [25] |
CHAUMONT J, SOULET S, KRUPA J C, et al. Competition between disorder creation and annealing in fluoroapatite nuclear waste forms. Journal of Nuclear Materials, 2002, 301(2): 122-128.
DOI URL |
| [26] |
MORENO E C, KRESAK M, ZAHRADNIK R T. Fluoridated hydroxyapatite solubility and caries formation. Nature, 1974, 247(5435): 64-65.
DOI URL |
| [27] |
GAO X N, HUANG Y, TENG Y C, et al. Fabrication and chemical durability of hot-pressed Na-bearing fluorapatite-type Ca8Sm1Na1(PO4)6F2 ceramic for immobilization of trivalent minor actinide. Journal of Nuclear Materials, 2018, 507: 297-305.
DOI URL |
| [28] |
OHNUKI T, KOZAI N, SAMADFAM M, et al. The formation of autunite (Ca(UO2)2(PO4)2•nH2O) within the leached layer of dissolving apatite: incorporation mechanism of uranium by apatite. Chemical Geology, 2004, 211(1): 1-14.
DOI URL |
| [29] |
FATHI M H, MOHAMMADI Z E. Mechanical alloying synthesis and bioactivity evaluation of nanocrystalline fluoridated hydroxyapatite. Journal of Crystal Growth, 2008, 311(5): 1392-1403.
DOI URL |
| [30] |
TROMMER R M, SANTOS L A, BERGMANN C P. Alternative technique for hydroxyapatite coatings. Surface & Coatings Technology, 2007, 201(24): 9587-9593.
DOI URL |
| [31] |
HAN M N, KONG L J, HU X L, et al. Phase migration and transformation of uranium in mineralized immobilization by wasted bio-hydroxyapatite. Journal of Cleaner Production, 2018, 197: 886-894.
DOI URL |
| [32] |
WOJCIECH P, WLADYSLAW R, ANITA P. Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Advances in Colloid and Interface Science, 2009, 152(1): 2-13.
DOI URL |
| [33] |
LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 1918, 40(9): 1361-1403.
DOI URL |
| [34] | FREUNDLICH H. Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie, 1906, 57: 385-471. |
| [35] |
ZHANG Z X, LIU H B, SONG W C, et al. Accumulation of U(VI) on the Pantoea sp. TW18 isolated from radionuclide-contaminated soils. Journal of Environmental Radioactivity, 2018, 192: 219-226.
DOI URL |
| [36] |
LI M X, LIU H B, CHEN T H, et al. Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Science of the Total Environment, 2018, 651(P1): 1020-1028.
DOI URL |
| [37] |
ZHENG N C, YIN L Y, SU M H, et al. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chemical Engineering Journal, 2020, 384: 123262
DOI URL |
| [1] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [2] | HUANG Xiubing, WANG Peng, TAO Jinzhang, XI Zuoshuai. CeO2 Modified Mn-Fe-O Composites and their Catalytic Performance for NH3-SCR of NO [J]. Journal of Inorganic Materials, 2020, 35(5): 573-580. |
| [3] | HUANG Xieyi,WANG Peng,YIN Guoheng,ZHANG Shaoning,ZHAO Wei,WANG Dong,BI Qingyuan,HUANG Fuqiang. Removal of Volatile Organic Compounds Driven by Platinum Supported on Amorphous Phosphated Titanium Oxide [J]. Journal of Inorganic Materials, 2020, 35(4): 482-490. |
| [4] | ZHANG Wei, LIU Chen, CHEN Yuantao, WU Wangsuo. Removal of Boron from Water by Mg-Al-Ce Hydrotalcite Adsorption [J]. Journal of Inorganic Materials, 2020, 35(3): 337-344. |
| [5] | SONG Huan, WANG Lin, WANG Hong-Qing, SHI Wei-Qun. Adsorption of Eu(III) on Alkalized Ti3C2Tx MXene Studied by Batch Experiment and Its Mechanism Investigation [J]. Journal of Inorganic Materials, 2020, 35(1): 65-72. |
| [6] | GENG Rui-Wen, YANG Xiao-Jing, XIE Qi-Ming, LI Rui, LUO Liang. Material Removal Mechanism of Monocrystalline Germanium Based on Nano-scratch Experiment [J]. Journal of Inorganic Materials, 2019, 34(8): 867-872. |
| [7] | XU Cong-Bin, YANG Wen-Jie, SUN Hong-Liang, LIU Wei-Jiang, YANG Yuan-Yu, LIN Ai-Jun. Performance and Mechanism of Pb(II) Removal by Expanded Graphite Loaded with Zero-Valent Iron [J]. Journal of Inorganic Materials, 2018, 33(1): 41-47. |
| [8] | WANG Yan-Li, WANG Xu-Jian, ZHAN Liang, QIAO Wen-Ming, LIANG Xiao-Yi, LING Li-Cheng. Structure Control of V2O5/CNFs/Cordierite Monolith Catalyst and Its Catalytic Performance on NO Removal from Flue Gas [J]. Journal of Inorganic Materials, 2012, 27(8): 800-806. |
| [9] |
LIU Shou-Xin,LIU Zheng-Feng.
Heterogeneous Photocatalytic Oxidation Removal of Gaseous Benzene over TiO2/ACF Composite Prepared by Improved Sol-Gel Method [J]. Journal of Inorganic Materials, 2009, 24(2): 209-214. |
| [10] | MA Xin-Pei,LI Guang-Xin,SHEN Lian,JIN Zhi-Hao. Cut Behavior and Microstructure of a Novel Mica Glass-ceramic [J]. Journal of Inorganic Materials, 2004, 19(1): 48-52. |
| [11] | YE Bin,CUI Kai,FENG Qing-Ling,CHEN Guo-Qiang,CUI Fu-Zhai. Synthesis and High Temperature Resistance Properties of Silver Loaded Fluorapatite Antibacterial [J]. Journal of Inorganic Materials, 2003, 18(2): 485-489. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||