
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (9): 931-941.DOI: 10.15541/jim20170585
• Orginal Article • Previous Articles Next Articles
ZHANG Peng, ZHANG Qing, LIU Jing, GAO Lian
Received:2017-12-07
Revised:2018-03-04
Published:2018-09-20
Online:2018-08-14
Supported by:CLC Number:
ZHANG Peng, ZHANG Qing, LIU Jing, GAO Lian. Research Progress of Ni-based Composite Catalysts for Methane Dry Reforming[J]. Journal of Inorganic Materials, 2018, 33(9): 931-941.
| Reaction | Reaction equation | △G | ΔH298/(kJ·mol-1) | Limit temperature/K |
|---|---|---|---|---|
| DRM | CH4+CO2→2H2+2CO | 61770-67.32T | 247.3 | 913 |
| RWGS | CO2+H2→CO+H2O | -8545+7.84T | 41.0 | 1093 |
| Boudouard reaction | 2CO→CO2+C | -39810+40.87T | -171.0 | 973 |
| Methane cracking | CH4→C+2H2 | 2190-26.45T | 75.0 | 830 |
Table 1 Main chemical reactions during methane dry reforming process[12,13]
| Reaction | Reaction equation | △G | ΔH298/(kJ·mol-1) | Limit temperature/K |
|---|---|---|---|---|
| DRM | CH4+CO2→2H2+2CO | 61770-67.32T | 247.3 | 913 |
| RWGS | CO2+H2→CO+H2O | -8545+7.84T | 41.0 | 1093 |
| Boudouard reaction | 2CO→CO2+C | -39810+40.87T | -171.0 | 973 |
| Methane cracking | CH4→C+2H2 | 2190-26.45T | 75.0 | 830 |
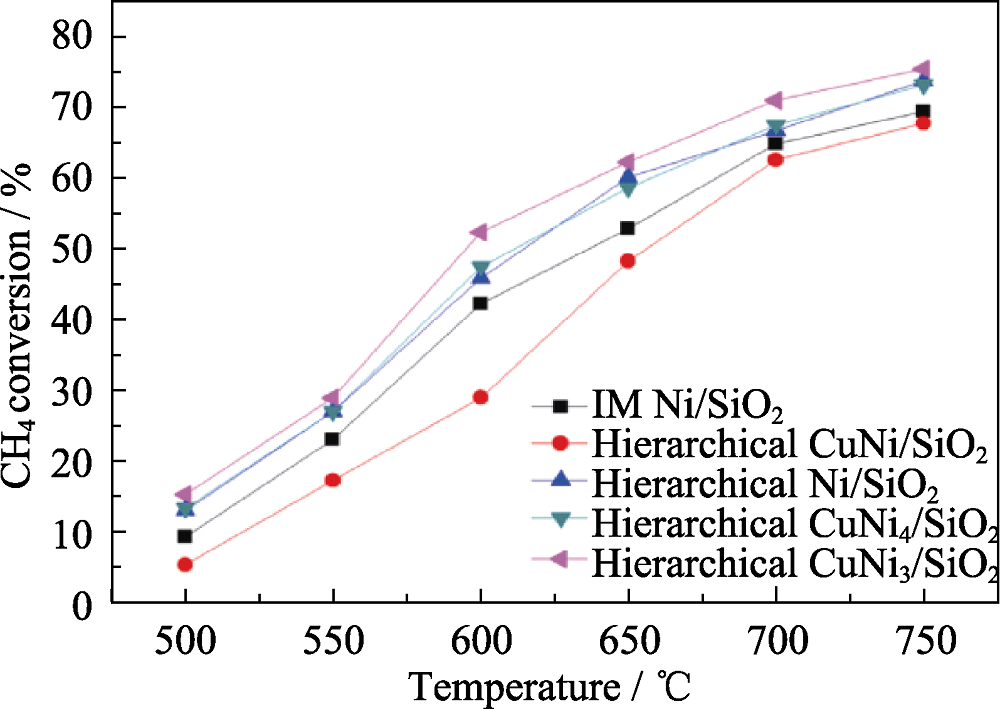
Fig. 3 Methane conversion of the methane dry reforming catalysis on the hierarchical Cu-Ni/SiO2 catalysts with various Cu/Ni ratios and the control sample IM Ni/SiO2 at different temperatures[22]
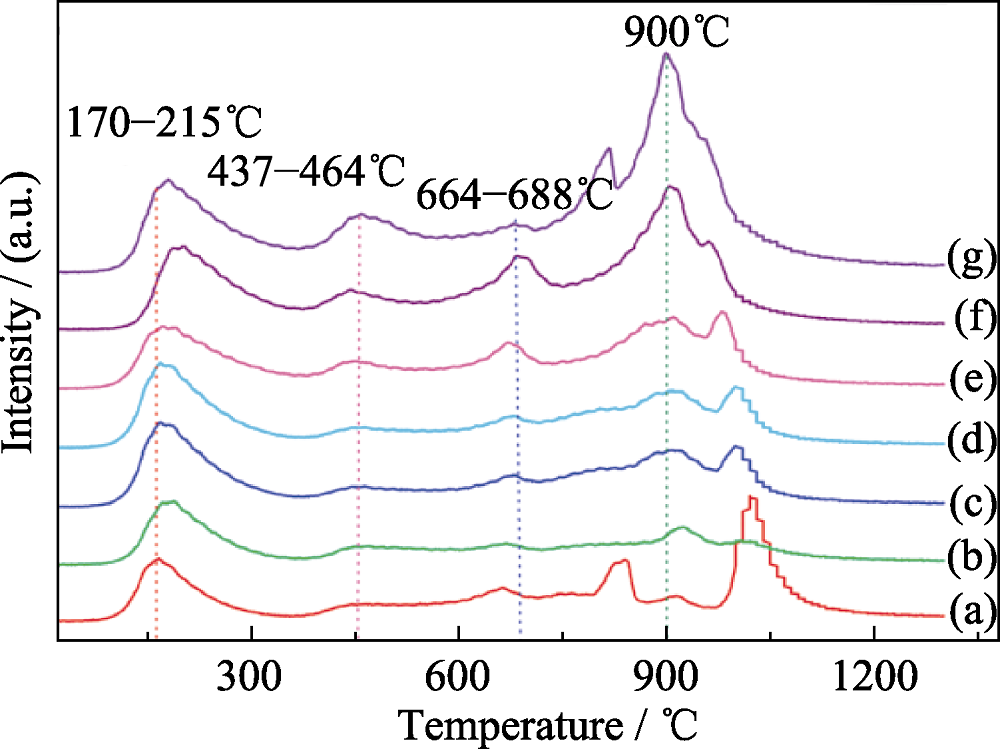
Fig. 4 CO2-TPD profiles of the M-5NixCa(95-x)Al materials calcined at 600℃ with different Ca contents[36] (a) M-5Ni95Al; (b) M-5Ni1Ca94Al; (c) M-5Ni2Ca93Al; (d) M-5Ni3Ca92Al; (e) M-5Ni5Ca90Al; (f) M-5Ni8Ca87Al; (g) M-5Ni10Ca85Al
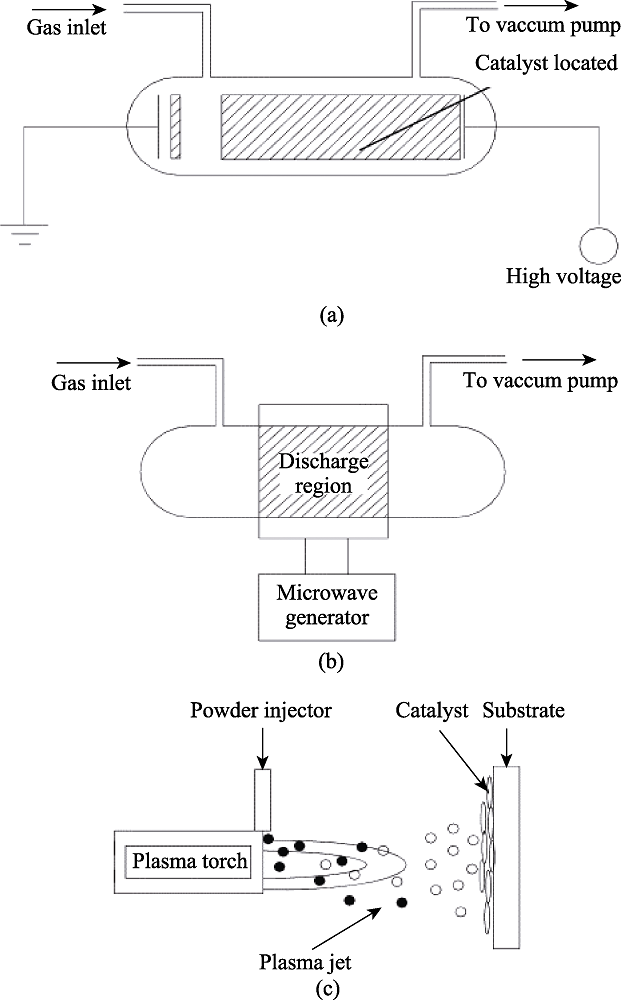
Fig. 9 Schematically representatives of electrode configurations of discharge phenomena applied for catalyst preparation[65](a) Glow discharge; (b) Microwave plasma; (c) Plasma spraying
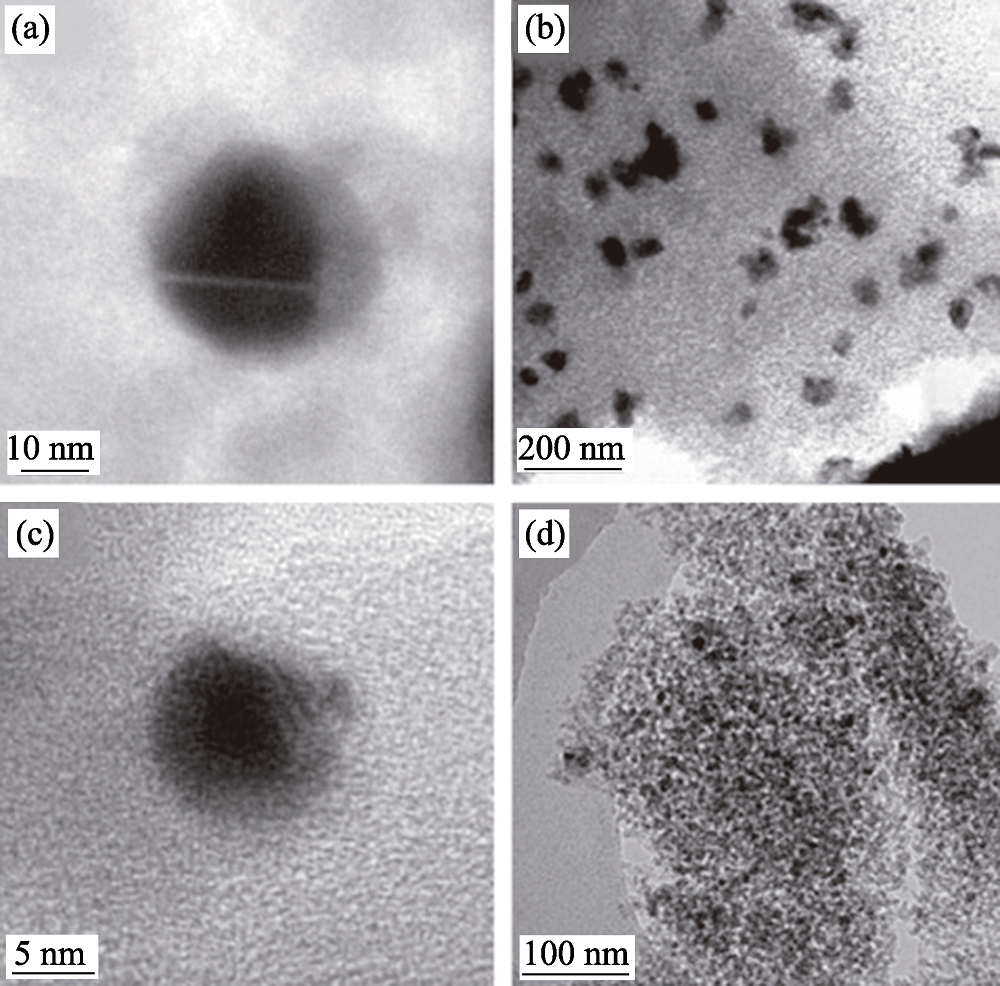
Fig. 10 TEM images of Ni/SiO2 catalyst samples prepared by two methods (a, b) the conventional catalyst and (c, d) the novel catalyst prepared by plasma jet[69]
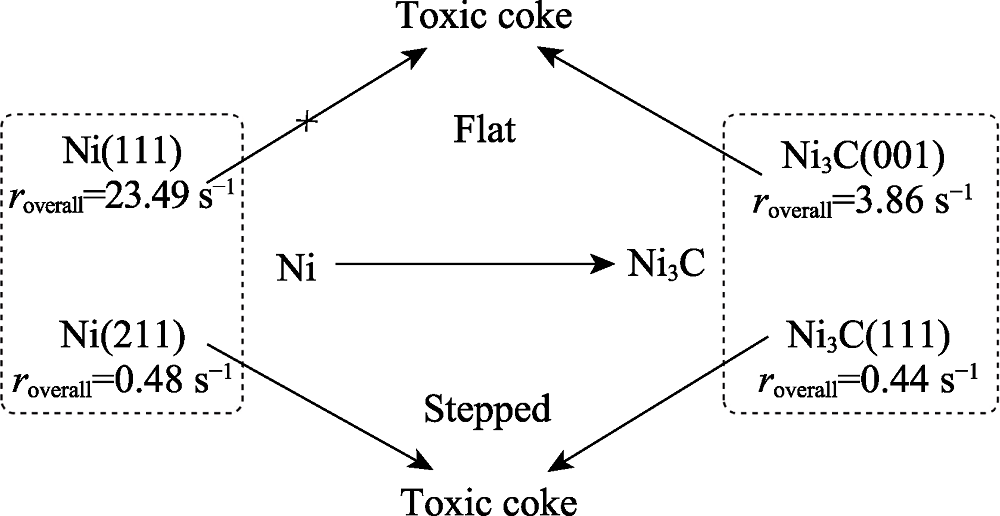
Fig. 11 Deactivation scheme to show schematically the phase transformation and coke formation[71]Ni(111) and Ni(211) stand for the clean nickel metal flat and stepped surfaces respectively, and Ni3C(001) and Ni3C(111) are the flat and stepped nickel carbide. Flat surfaces are marked in blue, while stepped surfaces are in red. Toxic coke is the more stable and high toxicity carbon atom which will result in the deactivation of the nickel catalyst. The corresponding overall reaction rates are marked below the different structures. The arrow with X means this step is not likely to occur, while arrow without X means this step could happen
| [1] | TOLLEFSON J.World looks ahead post-Copenhagen.Nature, 2009, 462(7276): 966-967. |
| [2] | TOLLEFSON J.Copenhagen: the scientists' view.Nature, 2009, 462(7274): 714. |
| [3] | WANG W, WANG S P, MA X B,et al. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev., 2011, 40(7): 3703-3727. |
| [4] | TOLLEFSON J.World’s carbon emissions set to spike by 2% in 2017.Nature News, 2017, 551(7680): 283. |
| [5] | SONG C S.Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing.Catal. Today, 2006, 115(1-4): 2-32. |
| [6] | MELIKOGLU M.Shale gas: analysis of its role in the global energy market.Renew Sust. Energ. Rev., 2014, 37: 460-468. |
| [7] | BERNARD S, HORSFIELD B.Thermal maturation of gas shale systems.Annu. Rev. Earth Planet. Sci., 2014, 42(1): 635-651. |
| [8] | WANG N, YU X, SHEN K,et al. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy, 2013, 38(23): 9718-9731. |
| [9] | SIBUDJING K, YASOTHA K, JUN N,et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem, 2015, 8(21): 3556-3575. |
| [10] | ASSABUMRUNGRAT S, CHAROENSERI S, LAOSIRIPOJANA N,et al. Effect of oxygen addition on catalytic performance of Ni/SiO·MgO toward carbon dioxide reforming of methane under periodic operation. Int. J. Hydrogen Energy, 2009, 34(15): 6211-6220. |
| [11] | KIM H Y, PARK J N, HENKELMAN G,et al. Design of a highly nanodispersed Pd-MgO/SiO2 composite catalyst with multifunctional activity for CH4 reforming. ChemSusChem, 2012, 5(8): 1474-1481. |
| [12] | FAN M S, ABDULLAH A Z, BHATIA S.Catalytic technology for carbon dioxide reforming of methane to synthesis gas.ChemCatChem, 2009, 1(2): 192-208. |
| [13] | WANG S, LU G Q, MILLAR G J.Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art. Energy & Fuels, 1996, 10(4): 896-904. |
| [14] | ZHANG J, WANG H, DALAI A K.Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal., 2007, 249(2): 300-310. |
| [15] | TOPALIDIS A, PETRAKIS D, LADAVOS A,et al. A kinetic study of methane and carbon dioxide interconversion over 0.5% Pt/SrTiO3 catalysts. Catal. Today, 2007, 127(1): 238-245. |
| [16] | PAKHARE D, SPIVEY J.A review of dry (CO2) reforming of methane over noble metal catalysts.Chem. Soc. Rev., 2014, 43(22): 7813-7837. |
| [17] | BRADFORD M C J, VANNICE M A. CO2 reforming of CH4 over supported Pt catalysts.J. Catal., 1998, 173(1): 157-171. |
| [18] | BIAN Z, SURYAWINATA I Y, KAWI S.Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane.Appl. Catal., B, 2016, 195: 1-8. |
| [19] | TSYGANOK A I, TSUNODA T, HAMAKAWA S,et al. Dry reforming of methane over catalysts derived from nickel-containing Mg-Al layered double hydroxides. J. Catal., 2003, 213(2): 191-203. |
| [20] | LIU C J, YE J, JIANG J,et al. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemSusChem, 2015, 3(3): 529-541. |
| [21] | ZHANG Q, WU T, ZHANG P,et al. Facile synthesis of hollow hierarchical Ni/g-Al2O3 nanocomposites for methane dry reforming catalysis. RSC Adv., 2014, 4(93): 51184-51193. |
| [22] | WU T, ZHANG Q, CAI W,et al. Phyllosilicate evolved hierarchical Ni- and Cu-Ni/SiO2 nanocomposites for methane dry reforming catalysis. Appl. Catal. A, 2015, 503: 94-102. |
| [23] | DAZA C E, GALLEGO J, MONDRAG N F,et al. High stability of Ce-promoted Ni/Mg-Al catalysts derived from hydrotalcites in dry reforming of methane. Fuel, 2010, 89(3): 592-603. |
| [24] | FERREIRA-APARICIO P, FERNANDEZ-GARCIA M, GUERRERO-RUIZ A,et al. Evaluation of the role of the metal-support interfacial centers in the dry reforming of methane on alumina- supported rhodium catalysts. J. Catal., 2000, 190(2): 296-308. |
| [25] | CHETTAPONGSAPHAN C, CHAROJROCHKUL S, ASSABUMRUNGRAT S,et al. Catalytic H2O and CO2 reforming of CH4 over perovskite-based La0.8Sr0.2Cr0.9Ni0.1O3: effects of pre-treatment and co-reactant/CH4 on its reforming characteristics. Appl. Catal., 2010, 386(1/2): 194-200. |
| [26] | GALLEGO G S, BATIOT-DUPEYRAT C, BARRAULT J,et al. Dry reforming of methane over LaNi1- yByO3±δ(B= Mg, Co) perovskites used as catalyst precursor. Appl. Catal. A, 2008, 334(1/2): 251-258. |
| [27] | GARC A-DI GUEZ M, PIETA I, HERRERA M,et al. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3. Catal. Today, 2010, 149(3/4): 380-387. |
| [28] | LUCR DIO A F, ASSAF J M, ASSAF E M. Methane conversion reactions on Ni catalysts promoted with Rh: influence of support.Appl. Catal. A, 2011, 400(1/2): 156-165. |
| [29] | HAN J W, KIM C, PARK J S,et al. Highly coke-resistant Ni nanoparticle catalysts with minimal sintering in dry reforming of methane. ChemSusChem, 2014, 7(2): 451-456. |
| [30] | ALIEH, KHALESI, HAMID, et al. Effects of lanthanum substitution by strontium and calcium in La-Ni-Al perovskite oxides in dry reforming of methane. Chin. J. Catal., 2008, 29(10): 960-968. |
| [31] | LI X, LI D, TIAN H,et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B, 2017, 202: 683-694. |
| [32] | LUNA A E C, IRIARTE M E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst.Appl. Catal. A, 2008, 343(1): 10-15. |
| [33] | XU Z, ZHEN M, BI Y,et al. Catalytic properties of Ni modified hexaaluminates LaNiyAl12-yO19-δ for CO2 reforming of methane to synthesis gas. Appl. Catal., A, 2000, 198(1): 267-723. |
| [34] | HU Y H.Solid-solution catalysts for CO2 reforming of methane.Catal. Today, 2009, 148(3): 206-211. |
| [35] | JAFARBEGLOO M, TARLANI A, MESBAH A W,et al. NiO-MgO Solid solution prepared by Sol-Gel method as precursor for Ni/MgO methane dry reforming catalyst: effect of calcination temperature on catalytic performance. Catal. Lett., 2016, 146(1): 238-248. |
| [36] | XU L, SONG H, CHOU L.One-pot synthesis of ordered mesoporous NiO-CaO-Al2O3 composite oxides for catalyzing CO2 reforming of CH4.ACS Catalysis, 2012, 2(7): 1331-1342. |
| [37] | ZUBENKO D, SINGH S, ROSEN B A.Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming.Appl. Catal. B, 2017, 209: 711-719. |
| [38] | LI Y, ZHANG Q, LIU J,et al. Corona shaped SiO2 supported Ni nanoparticles for methane dry reforming catalysis. J. Chin. Ceram. Soc., 2015, 43(7): 911-918. |
| [39] | BIAN Z, DAS S, WAI M H,et al. A review on bimetallic Ni-based catalysts for CO2 reforming of methane. ChemPhysChem A, 2017, 18(22): 3117-3134 |
| [40] | TU W, GHOUSSOUB M, SINGH C V,et al. Consequences of surface oxophilicity of Ni, Ni-Co, and Co clusters on methane activation. J. Am. Chem. Soc., 2017, 139(20): 6928-6945. |
| [41] | CHEN Y G, YAMAZAKI O, TOMISHIGE K,et al. Noble metal promoted Ni0.03Mg0.97O solid solution catalysts for the reforming of CH4 with CO2. Catal. Lett., 1996, 39(1/2): 91-95. |
| [42] | WU H, PANTALEO G, PAROLA V L, ,et al. Bi-. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MOx (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B, 2014, 156-157(2): 350-361. |
| [43] | HORIUCHI T, SAKUMA K, FUKUI T,et al. Suppression of carbon deposition in the CO2 -reforming of CH4 by adding basic metal oxides to a Ni/Al2O3 catalyst. Appl. Catal. A, 1996, 144(1/2): 111-120. |
| [44] | OSAKI T, MORI T.Role of potassium in carbon-free CO2 reforming of methane on K-promoted Ni/Al2O3 catalysts.J. Catal., 2001, 204(1): 89-97. |
| [45] | FRUSTERI F, SPADARO L, ARENA F,et al. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni/MgO catalysts during the dry reforming of methane. Carbon, 2002, 40(7): 1063-1070. |
| [46] | DIAS J A C, ASSAF J M. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane. Catal. Today, 2003, 85(1): 59-68. |
| [47] | KOO K Y, ROH H S, YU T S,et al. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A, 2008, 340(2): 183-190. |
| [48] | KATHIRASER Y, THITSARTARN W, SUTTHIUMPORN K,et al. Inverse NiAl2O4 on LaAlO3-Al2O3: unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C, 2013, 117(16): 8120-8130. |
| [49] | SUTTHIUMPORN K, KAWI S.Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: role of surface oxygen species on H2 production and carbon suppression.Int. J. Hydrogen Energy, 2011, 36(22): 14435-14446. |
| [50] | STAGG-WILLIAMS S M, FENDLEY G, RESASCO D E,et al. CO2 reforming of CH4 over Pt/ZrO2 catalysts promoted with La and Ce oxides. J. Catal., 2000, 194(2): 240-249. |
| [51] | VALENTINI A, PROBST L F D, LISBOA-FILHO P N, et al. Role of vanadium in Ni:Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. A, 2003, 255(2): 211-220. |
| [52] | PAN Y, KUAI P, LIU Y,et al. Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Ni catalyst. Energy Environ. Sci., 2010, 3(9): 1322-1325. |
| [53] | HOU Z, YOKOTA O, TANAKA T,et al. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A, 2003, 253(2): 381-387. |
| [54] | GUO J, LOU H, ZHAO H,et al. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A, 2004, 273(1): 75-82. |
| [55] | MARGOSSIAN T, LARMIER K, KIM S M,et al. Molecularly- tailored nickel precursor and support yield a stable methane dry reforming catalyst with superior metal utilization. J. Am. Chem. Soc., 2017, 139(20): 6919-6927. |
| [56] | LIU S, BAI S Q, ZHENG Y,et al. Composite metal-oxide nanocatalysts. ChemCatChem, 2012, 4(10): 1462-1484. |
| [57] | ZHANG J, LI F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B, 2015, s176-177: 513-521. |
| [58] | KIM D H, KIM S Y, HAN S W,et al. The catalytic stability of TiO2-shell/Ni-core catalysts for CO2 reforming of CH4. Appl. Catal. A, 2015, 495: 184-191. |
| [59] | BAKTASH E, LITTLEWOOD P, SCHOM CKER R,et al. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B, 2015, 179: 122-127. |
| [60] | LIM Z Y, WU C, WANG W G,et al. Porosity effect on ZrO2 hollow shells and hydrothermal stability for catalytic steam reforming of methane. Journal of Materials Chemistry A, 2015, 4(1): 153-159. |
| [61] | WU T, CAI W, ZHANG P,et al. Cu-Ni@SiO2 alloy nanocomposites for methane dry reforming catalysis. RSC Adv., 2013, 3(46): 23976-23979. |
| [62] | DU X, ZHANG D, SHI L,et al. Coke- and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale, 2013, 5(7): 2659-2663. |
| [63] | LI W, ZHAO Z, JIAO Y.Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets.Int. J. Hydrogen Energy, 2016, 41(40): 17907-17921. |
| [64] | ZHU X, HUO P, ZHANG Y P,et al. Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl. Catal. B, 2008, 81(1/2): 132-140. |
| [65] | LIU C J, VISSOKOV G P, JANG W L.Catalyst preparation using plasma technologies.Catal. Today, 2002, 72(3/4): 173-184. |
| [66] | LIU C J.Plasma application for more environmentally friendly catalyst preparation. Pure Appl. Chem., 2006, 78(6): 1227-1238. |
| [67] | HONG J, CHU W, CHERNAVSKII P A,et al. Cobalt species and cobalt-support interaction in glow discharge plasma-assisted Fischer- Tropsch catalysts. J. Catal., 2010, 273(1): 9-17. |
| [68] | CHENG D G, ZHU X, BEN Y,et al. Carbon dioxide reforming of methane over Ni/Al2O3 treated with glow discharge plasma. Catal. Today, 2006, 115(1-4): 205-210. |
| [69] | LIU G, LI Y, CHU W,et al. Plasma-assisted preparation of Ni/SiO2 catalyst using atmospheric high frequency cold plasma jet. Catal. Commun., 2008, 9(6): 1087-1091. |
| [70] | WANG S G, CAO D B, LI Y W,et al. CO2 reforming of CH4 on Ni (111): a density functional theory calculation. J. Phys. Chem. B, 2006, 110(20): 9976-9983. |
| [71] | WANG Z, CAO X M, ZHU J,et al. Activity and coke formation of nickel and nickel carbide in dry reforming: a deactivation scheme from density functional theory. J. Catal., 2014, 311(3): 469-480. |
| [72] | AW M S, ZORKO M, DJINOVIĆ P,et al. Insights into durable NiCo catalysts on β-SiC/CeZrO2 and γ-Al2O3/CeZrO2 advanced supports prepared from facile methods for CH4-CO2 dry reforming. Appl. Catal. B, 2015, 164: 100-112. |
| [73] | OEMAR U, HIDAJAT K, KAWI S.Role of catalyst support over PdO-NiO catalysts on catalyst activity and stability for oxy-CO2 reforming of methane.Appl. Catal. A, 2011, 402(1/2): 176-187. |
| [74] | NI J, CHEN L, LIN J,et al. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy, 2012, 1(5): 674-686. |
| [75] | HAN J, ZHAN Y, STREET J,et al. Natural gas reforming of carbon dioxide for syngas over Ni-Ce-Al catalysts. Int. J. Hydrogen Energy, 2017, 42(29): 18364-18374. |
| [76] | CHEN Q, ZHANG J, PAN B,et al. Temperature-dependent anti-coking behaviors of highly stable Ni-CaO-ZrO2 nanocomposite catalysts for CO2 reforming of methane. Chem. Eng. J., 2017, 320: 63-73. |
| [77] | THEOFANIDIS S A, GALVITA V V, SABBE M,et al. Controlling the stability of a Fe-Ni reforming catalyst: structural organization of the active components. Appl. Catal. B, 2017, 209: 405-416. |
| [1] | WANG Hai-Long, WANG Yang, WANG Xiang-Wei, ZHANG Hong-Zhi. Research Progress of Thermal Controlled Cracking of Hard-Brittle Plate [J]. Journal of Inorganic Materials, 2018, 33(9): 923-930. |
| [2] | LI Jiang, DAI Jia-Wei, PAN Yu-Bai. Research Progress on Magneto-optical Transparent Ceramics [J]. Journal of Inorganic Materials, 2018, 33(1): 1-8. |
| [3] | ZHENG Yan-Bin, JIANG Zhi-Gang, ZHU Pin-Wen. Development on the Preparation and Application of Onion-like Carbon [J]. Journal of Inorganic Materials, 2015, 30(8): 793-801. |
| [4] | LIU Gang, YAN Yan. Research Progress of Porous Ceramics Produced by Freeze Casting Technique [J]. Journal of Inorganic Materials, 2014, 29(6): 571-583. |
| [5] | YANG Pei-Zhi,LIU Li-Ming,ZHANG Xiao-Wen,MO Jing-Hui. Research Progress of Long-wavelength Infrared Optical Materials [J]. Journal of Inorganic Materials, 2008, 23(4): 641-646. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||