
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (11): 1245-1253.DOI: 10.15541/jim20240218
Special Issue: 【信息功能】敏感陶瓷(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
DING Ningning1,2( ), SUN Jianhua1,2, WEI Xu1,2, SUN Lixia1,2(
), SUN Jianhua1,2, WEI Xu1,2, SUN Lixia1,2( )
)
Received:2024-04-26
Revised:2024-06-24
Published:2024-11-20
Online:2024-07-16
Contact:
SUN Lixia, associate professor. E-mail: binglin0628@163.comAbout author:DING Ningning (1997-), female, Master candidate. E-mail: dingning1585@163.com
Supported by:CLC Number:
DING Ningning, SUN Jianhua, WEI Xu, SUN Lixia. Monitoring Ammonia at Room Temperature of p-Aminobenzene Sulfonic Acid Modified MoO3/PPy Composites[J]. Journal of Inorganic Materials, 2024, 39(11): 1245-1253.
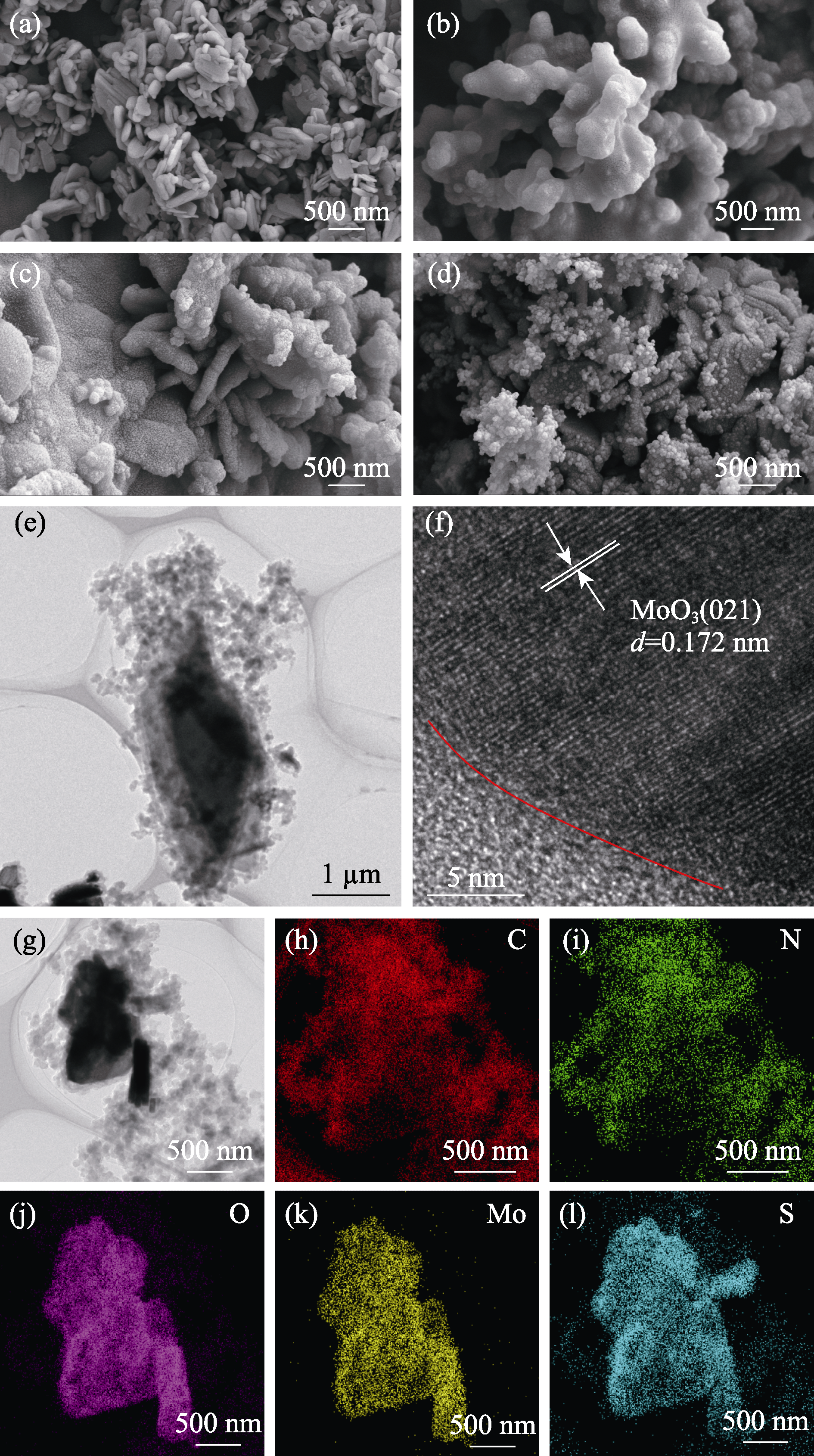
Fig. 4 Electron microscopy and surface element scanning of samples (a-d) SEM images of (a) MoO3, (b) PPy, (c) MP10 and (d) MPA40; (e) TEM and (f) HRTEM images of MPA40; (g-l) Element mappings of MPA40
| Sample | Zeta potential/mV |
|---|---|
| PPy | -13 |
| MP10 | -18 |
| MPA20 | -25 |
| MPA30 | -27 |
| MPA40 | -33 |
| MPA50 | -30 |
| MPA60 | -30 |
Table 1 Zeta potential of the materials
| Sample | Zeta potential/mV |
|---|---|
| PPy | -13 |
| MP10 | -18 |
| MPA20 | -25 |
| MPA30 | -27 |
| MPA40 | -33 |
| MPA50 | -30 |
| MPA60 | -30 |
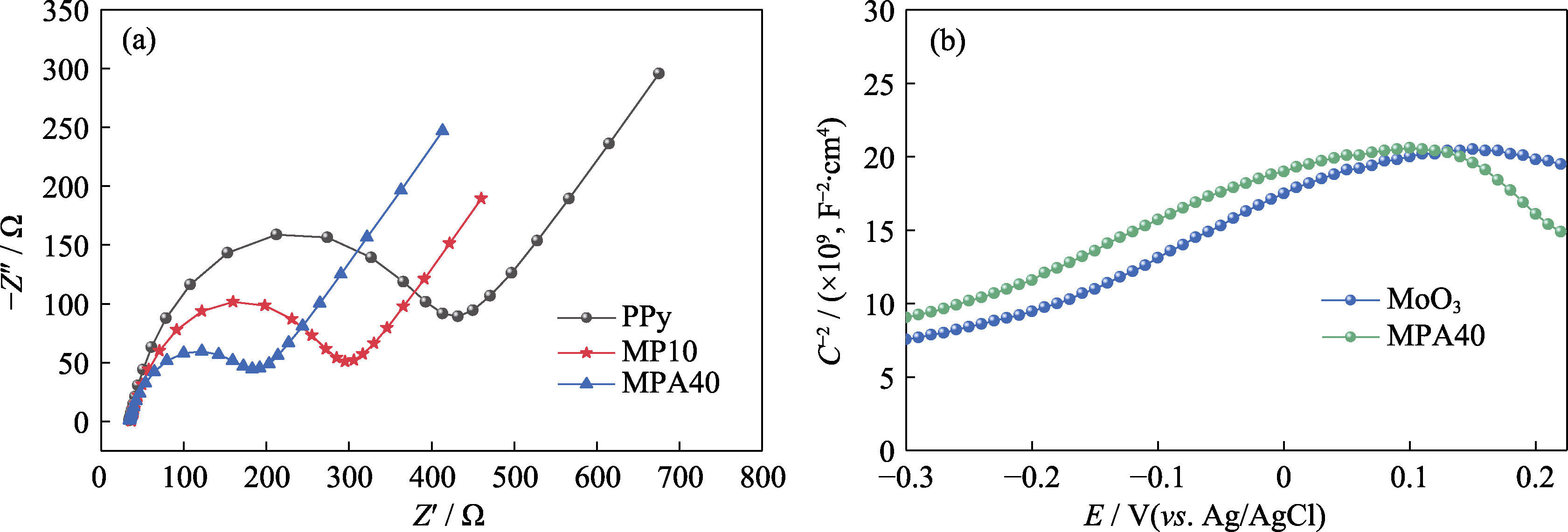
Fig. 5 Electrical properties tests of samples (a) EIS plots of PPy, MP10 and MPA40; (b) Mott-Schottky plots of MoO3 and MPA40; Colorful figures are available on website
| Material | T/℃ | Mass concentration/ (µg·L-1) | Response/% | Ref. |
|---|---|---|---|---|
| PPy | RT | 1×105 | 34.7 | [ |
| PPy-V2O5-MnO2 | RT | 0.2×105 | 45.67 | [ |
| MXene/MoS2/PPy | RT | 0.1×105 | 108 | [ |
| PPy/CeO2 | RT | 50×105 | 93.4 | [ |
| NiO/PPy | RT | 45 | 65 | [ |
| rGO-PPy-SnO2 | RT | 0.1×105 | 53 | [ |
| PPy-ZnO-CSA | RT | 1.2×105 | 79 | [ |
| pABSA-MoO3/PPy | RT | 1×105 | 188 | This work |
Table 2 Ammonia sensing properties of different materials
| Material | T/℃ | Mass concentration/ (µg·L-1) | Response/% | Ref. |
|---|---|---|---|---|
| PPy | RT | 1×105 | 34.7 | [ |
| PPy-V2O5-MnO2 | RT | 0.2×105 | 45.67 | [ |
| MXene/MoS2/PPy | RT | 0.1×105 | 108 | [ |
| PPy/CeO2 | RT | 50×105 | 93.4 | [ |
| NiO/PPy | RT | 45 | 65 | [ |
| rGO-PPy-SnO2 | RT | 0.1×105 | 53 | [ |
| PPy-ZnO-CSA | RT | 1.2×105 | 79 | [ |
| pABSA-MoO3/PPy | RT | 1×105 | 188 | This work |
| [1] | GUPTA V, MALIK R, KUMAR L. Highly efficient and cost- effective polyaniline-based ammonia sensor on the biodegradable paper substrate at room temperature. Materials Chemistry and Physics, 2023, 310: 128388. |
| [2] | LI P P, WANG B, QIN C, et al. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceramics International, 2020, 46(11): 19232. |
| [3] |
KWAK D, LEI Y, MARIC R. Ammonia gas sensors: a comprehensive review. Talanta, 2019, 204: 713.
DOI PMID |
| [4] | YUAN K P, ZHU L Y, YANG J H, et al. Precise preparation of WO3@SnO2 core shell nanosheets for efficient NH3 gas sensing. Journal of Colloid and Interface Science, 2020, 568: 81. |
| [5] | GAUTAM S K, PANDA S. Highly sensitive Cu-ethylenediamine/ PANI composite sensor for NH3 detection at room temperature. Talanta, 2023, 258: 124418. |
| [6] | NAKATE U T, BHUYAN P, YU Y T, et al. Synthesis and characterizations of highly responsive H2S sensor using p-type Co3O4 nanoparticles/nanorods mixed nanostructures. International Journal of Hydrogen Energy, 2022, 47(12): 8145. |
| [7] | WEN X H, SUN J H, SUN L X, et al. Preparation and acetone sensing properties of CuO-CeO2 nanocomposites with p-n heterostructures. Fine Chemistry, 2021, 38(4): 736. |
| [8] | ANANDA S R, KUMARI L, MURUGENDRAPPA M V. Studies on room-temperature acetone sensing properties of ZnCo2O4/PPy and MnCo2O4/PPy nanocomposites for diabetes diagnosis. Applied Physics A, 2022, 128(8): 669. |
| [9] |
SETKA M, CALAVIA R, VOJKUVKA L, et al. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Scientific Reports, 2019, 9: 8465.
DOI PMID |
| [10] | GAO L, YIN C Q, LUO Y Y, et al. Facile synthesis of the composites of polyaniline and TiO2 nanoparticles using self-assembly method and their application in gas sensing. Nanomaterials, 2019, 9(4): 493. |
| [11] | SETKA M, BAHOS F A, MATATAGUI D, et al. Love wave sensors based on gold nanoparticle-modified polypyrrole and their properties to ammonia and ethylene. Sensors and Actuators B: Chemical, 2020, 304: 127337. |
| [12] | SOOD Y, PAWAR V S, MUDILA H, et al. A review on synthetic strategies and gas sensing approach for polypyrrole-based hybrid nanocomposites. Polymer Engineering and Science, 2021, 61(12): 2949. |
| [13] | ZHANG D Z, WU Z L, ZONG X Q, et al. Fabrication of polypyrrole/ Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sensors and Actuators B: Chemical, 2018, 274: 575. |
| [14] | KAUR A, KUMAR R. Sensing of ammonia at room temperature by polypyrrole-tin oxide nanostructures: investigation by Kelvin probe force microscopy. Sensors and Actuators A: Physical, 2016, 245: 113. |
| [15] | JAIN S, KARMAKAR N, SHAH A, et al. Ammonia detection of 1-D ZnO/polypyrrole nanocomposite: effect of CSA doping and their structural, chemical, thermal and gas sensing behavior. Applied Surface Science, 2017, 396: 1317. |
| [16] | MA Y Z, ZHANG S, WANG Q, et al. Ag-decorated MoO3 microspheres gas sensor for triethylamine detection with rapid response/recovery. Inorganic Chemistry Communications, 2023, 157: 111442. |
| [17] | WANG C, YANG M, LIU L H, et al. One-step synthesis of polypyrrole/Fe2O3 nanocomposite and the enhanced response of NO2 at low temperature. Journal of Colloid and Interface Science, 2020, 560: 312. |
| [18] | MANE A T, NAVALE S T, PATIL V B. Room temperature NO2 gas sensing properties of DBSA doped PPy-WO3 hybrid nanocomposite sensor. Organic Electronics, 2015, 19: 15. |
| [19] | SOBHANI-NASAB A, BEHVANDI S, KARIMI M A, et al. Synergetic effect of graphene oxide and C3N4 as co-catalyst for enhanced photocatalytic performance of dyes on Yb2(MoO4)3/ YbMoO4 nanocomposite. Ceramics International, 2019, 45(14): 17847. |
| [20] | NEISI Z, ANSARI-ASL Z, JAFARINEJAD-FARSANGI S, et al. Synthesis, characterization and biocompatibility of polypyrrole/ Cu(II) metal-organic framework nanocomposites. Colloids and Surfaces B: Biointerfaces, 2019, 178: 365. |
| [21] | ZHOU K F, SHEN D F, LI X, et al. Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta, 2020, 209: 120507. |
| [22] | NALAGE S R, NAVALE S T, MANE R S, et al. Preparation of camphor-sulfonic acid doped PPy-NiO hybrid nanocomposite for detection of toxic nitrogen dioxide. Synthetic Metals, 2015, 209: 426. |
| [23] |
DUONG N H, NGUYEN T T, TRAN D V, et al. Synergistic effects in the gas sensitivity of polypyrrole/single wall carbon nanotube composites. Sensors, 2012, 12(6): 7965.
DOI PMID |
| [24] | IDRIS N H, WANG J Z, CHOU S L, et al. Effects of polypyrrole on the performance of nickel oxide anode materials for rechargeable lithium-ion batteries. Journal of Materials Research, 2011, 26(7): 860. |
| [25] | JIAO Y, CHEN G, CHEN D H, et al. Bimetal-organic framework assisted polymerization of pyrrole involving air oxidant to prepare composite electrodes for portable energy storage. Journal of Materials Chemistry A, 2017, 5(45): 23744. |
| [26] | ZAKHAROVA G S, SCHMIDT C, OTTMANN A, et al. Microwave-assisted hydrothermal synthesis and electrochemical studies of α- and h-MoO3. Journal of Solid State Electrochemistry, 2018, 22(12): 3651. |
| [27] | RAUT B T, CHOUGULE M A, GHANWAT A A, et al. Polyaniline-CdS nanocomposites: effect of camphor sulfonic acid doping on structural, microstructural, optical and electrical properties. Journal of Materials Science-Materials in Electronics, 2012, 23(12): 2104. |
| [28] | WEI Z Q, HOU S, LIN X, et al. Unexpected boosted solar water oxidation by nonconjugated polymer-mediated tandem charge transfer. Journal of the American Chemical Society, 2020, 142(52): 21899. |
| [29] | YU W L, LI F, HUANG T, et al. Go beyond the limit: rationally designed mixed-dimensional perovskite/semiconductor heterostructures and their applications. Innovation, 2023, 4(1): 100363. |
| [30] | HAN Z Z, WANG J J, LIAO L, et al. Phosphorus doped TiO2 as oxygen sensor with low operating temperature and sensing mechanism. Applied Surface Science, 2013, 273: 349. |
| [31] | HONGSITH N, CHANSURIYA S, KOENROBKET S, et al. Investigating of transition state on the Pd-Au decorated ZnO nanoparticle layers for gas sensor application. Heliyon, 2023, 9(9): 19402. |
| [32] | CHANG J N, ZHANG H J, CAO J L, et al. Ultrahigh sensitive and selective triethylamine sensor based on h-BN modified MoO3 nanowires. Advanced Powder Technology, 2022, 33(2): 103432. |
| [33] | IKRAM M, LIU L J, LV H, et al. Intercalation of Bi2O3/Bi2S3 nanoparticles into highly expanded MoS2 nanosheets for greatly enhanced gas sensing performance at room temperature. Journal of Hazardous Materials, 2019, 363: 335. |
| [34] | YU J G, YUE L, LIU S W, et al. Hydrothermal preparation and photocatalytic activity of mesoporous Au-TiO2 nanocomposite microspheres. Journal of Colloid and Interface Science, 2009, 334(1): 58. |
| [35] | ZHAO H Y, SUN J H, LIU J M, et al. UV-triggered carrier transport regulation of fibrous NiO/SnO2 heterostructures for triethylamine detection. Chemical Engineering Journal, 2023, 476: 146687. |
| [36] | LI X, SUN L J, YANG X Y, et al. Enhancing the colorimetric detection of H2O2 and ascorbic acid on polypyrrole coated fluconazole-functionalized POMOFs. Analyst, 2019, 144(10): 3347. |
| [37] | ZHANG J, WU C Y, LI T, et al. Highly sensitive and ultralow detection limit of room-temperature NO2 sensors using in-situ growth of PPy on mesoporous NiO nanosheets. Organic Electronics, 2020, 77: 105504. |
| [38] | QIAO T T, WANG G S, SHEN Y B, et al. Rational design of CuO/In2O3 heterostructures with flower-like structures for low temperature detection of formaldehyde. Journal of Alloys and Compounds, 2022, 896: 16959. |
| [39] | LIU J Y, DAI M J, WANG T S, et al. Enhanced gas sensing properties of SnO2 hollow spheres decorated with CeO2 nanoparticles heterostructure composite materials. ACS Applied Materials & Interfaces, 2016, 8(10): 6669. |
| [40] | ANDREOLI E, ROONEY D A, REDINGTON W, et al. Electrochemical deposition of hierarchical micro/nanostructures of copper hydroxysulfates on polypyrrole-polystyrene sulfonate films. Journal of Physical Chemistry C, 2011, 115(17): 8725. |
| [41] | KARMAKAR N, FERNANDES R, JAIN S, et al. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sensors and Actuators B: Chemical, 2017, 242: 118. |
| [42] | XU L, GE M Y, ZHANG F, et al. Nanostructured of SnO2/NiO composite as a highly selective formaldehyde gas sensor. Journal of Materials Research, 2020, 35(22): 3079. |
| [43] | LAWANIYA S D, KUMAR S, YU Y, et al. Ammonia sensing properties of PPy nanostructures (urchins/flowers) towards low-cost and flexible gas sensors at room temperature. Sensors and Actuators B: Chemical, 2023, 382: 133566. |
| [44] | MALOOK K, KHAN H, SHAH M. Ammonia sensing behavior of polypyrrole-bimetallic oxide composites. Polymer Composites, 2020, 41(7): 2610. |
| [45] | LU L, LIU M Y, SUI Q L, et al. MXene/MoS2 nanosheet/ polypyrrole for high-sensitivity detection of ammonia gas at room temperature. Materials Today Communications, 2023, 35: 106239. |
| [46] | HUSAIN A, AL-ZAHRANI S A, AL OTAIBI A, et al. Fabrication of reproducible and selective ammonia vapor sensor-pellet of polypyrrole/cerium oxide nanocomposite for prompt detection at room temperature. Polymers, 2021, 13(11): 1829. |
| [47] | HIEN H T, THU D T A, NGAN P Q, et al. High NH3 sensing performance of NiO/PPy hybrid nanostructures. Sensors and Actuators B: Chemical, 2021, 340: 129986. |
| [48] | AMITH S L, GURUNATHAN K. Active sites tailored rGO-PPy nanosheets with high crystalline tetragonal SnO2 nanocrystals for ammonia e-sensitization at room temperature. Journal of Alloys and Compounds, 2023, 960: 170819. |
| [49] | SHEN C Y, HUNG T T, CHUANG Y W, et al. Room-temperature NH3 gas surface acoustic wave (SAW) sensors based on graphene/PPy composite films decorated by Au nanoparticles with ppb detection ability. Polymers, 2023, 15(22): 4353. |
| [50] | PATOWARY B B, LASKAR S, NARZARY R, et al. Synthesis, characterization, and study of NO2 gas sensing behavior of CSA doped PANi-Ta2O5 nanocomposite. IEEE Sensors Journal, 2020, 20(7): 3429. |
| [1] | LIAN Minli, SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin. Supported Ni Catalysts from Ni-Mg-Al Hydrotalcite-like Compounds:Preparation and Catalytic Performance for Ammonia Decomposition [J]. Journal of Inorganic Materials, 2025, 40(1): 53-60. |
| [2] | YANG Xin, HAN Chunqiu, CAO Yuehan, HE Zhen, ZHOU Ying. Recent Advances in Electrocatalytic Nitrate Reduction to Ammonia Using Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(9): 979-991. |
| [3] | ZHANG Xiangsong, LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi, YANG Juan. Self-assembled Platinum-iridium Alloy Aerogels and Their Efficient Electrocatalytic Ammonia Oxidation Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 511-520. |
| [4] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [5] | LI Tao, CAO Pengfei, HU Litao, XIA Yong, CHEN Yi, LIU Yuejun, SUN Aokui. NH4+ Assisted Interlayer-expansion of MoS2: Preparation and Its Zinc Storage Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 79-86. |
| [6] | LI Wenbo, QIAN Rong, ZHUO Shangjun, JIANG Hong, SHENG Cheng, ZHU Yueqin. MoS2 with Different Morphologies: Preparation and Gas-sensing Property of NH3 [J]. Journal of Inorganic Materials, 2022, 37(10): 1135-1140. |
| [7] | CHU Yuxing, LIU Hairui, YAN Shuang. Preparation and Gas Sensing Properties of SnO2/NiO Composite Semiconductor Nanofibers [J]. Journal of Inorganic Materials, 2021, 36(9): 950-958. |
| [8] | ZHANG Yiqing,ZHANG Shujuan,WAN Zhengrui,MO Han,WANG Niangui,ZHOU Liqun. RuFe Nanoparticles Modified Sheet-like BiVO4 : High-efficient Synergistic Catalyst for Ammonia Borane Hydrolytic Dehydrogenation [J]. Journal of Inorganic Materials, 2020, 35(7): 809-816. |
| [9] | WU Fan, ZHAO Ziyan, LI Bangxin, DONG Fan, ZHOU Ying. Interfacial Oxygen Vacancy of Bi2O2CO3/PPy and its Visible-light Photocatalytic NO Oxidation Mechanism [J]. Journal of Inorganic Materials, 2020, 35(5): 541-548. |
| [10] | SUI Li-Li, WANG Run, ZHAO Dan, SHEN Shu-Chang, SUN Li, XU Ying-Ming, CHENG Xiao-Li, HUO Li-Hua. Construction of Hierarchical α-MoO3 Hollow Microspheres and Its High Adsorption Performance towards Organic Dyes [J]. Journal of Inorganic Materials, 2019, 34(2): 193-200. |
| [11] | ZHANG Yu-Hui, YI Qing-Feng, LIU Xiao-Ping, XIANG Bai-Lin. Carbonizing Products of the Fe/Co Doped Polypyrrole as Efficient Electrocatalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2014, 29(3): 269-274. |
| [12] | LIU Jian-Hua, ZHANG Shi-Lu, YU Mei, AN Jun-Wei, LI Song-Mei. Synthesis and Capacitance Characteristics of the Graphene Grafted Polypyrrole Composites [J]. Journal of Inorganic Materials, 2013, 28(4): 403-408. |
| [13] | XIAO Yuan-Hua, TANG Xin-Cun, WANG Zhi-Min, LI Feng, CHEN Gu-Chun, LI Lian-Xing, ZHANG Liang. Hierarchical PANI/MWCNT Nanocomposite: Synthesis, Characterization and Gas Sensing Properties [J]. Journal of Inorganic Materials, 2010, 25(10): 1092-1098. |
| [14] | XIONG Hao-Yang,HU Bin-Bin,XUE Zhong-Hui,CAI Li,DAI Shu-Xi,DU Zu-Liang. Growth of PbS Crystals under a BSA Monolayer in the Presence of Kinetically Controlled Ammonia Diffusion [J]. Journal of Inorganic Materials, 2010, 25(1): 63-67. |
| [15] | WEN Yi-Yun,LIU Zhi-Ming,CAI Li,GUO Jia-Xiu,GONG Mao-Chu,CHEN Yao-Qiang. Preparation of Pt/MoO3/ZrO2 Catalyst and its Property of Three-Way Catalysts [J]. Journal of Inorganic Materials, 2008, 23(6): 1267-1271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||