无机材料学报 ›› 2025, Vol. 40 ›› Issue (11): 1268-1276.DOI: 10.15541/jim20250009 CSTR: 32189.14.jim20250009
王红琴1,2( ), 邓浩2, 梁华1, 田强1, 晏敏皓1, 黄毅1(
), 邓浩2, 梁华1, 田强1, 晏敏皓1, 黄毅1( )
)
收稿日期:2025-01-07
修回日期:2025-03-10
出版日期:2025-11-20
网络出版日期:2025-04-02
通讯作者:
黄 毅, 讲师. E-mail: huangyi516@163.com作者简介:王红琴(1999-), 女, 硕士研究生. E-mail: 1523555457@qq.com
基金资助:
WANG Hongqin1,2( ), DENG Hao2, LIANG Hua1, TIAN Qiang1, YAN Minhao1, HUANG Yi1(
), DENG Hao2, LIANG Hua1, TIAN Qiang1, YAN Minhao1, HUANG Yi1( )
)
Received:2025-01-07
Revised:2025-03-10
Published:2025-11-20
Online:2025-04-02
Contact:
HUANG Yi, lecturer. E-mail: huangyi516@163.comAbout author:WANG Hongqin (1999-), female, Master candidate. E-mail: 1523555457@qq.com
Supported by:摘要:
天然铀矿的开采过程会产生含铀废液, 从含铀废液中去除铀(VI)已成为核工业亟待解决的关键问题。本研究基于“源于铀矿, 归于铀矿”的核心理念, 选择正钒酸钙(Ca3(VO4)2)作为去除铀(VI)的吸附材料, 系统研究了在不同吸附条件下, Ca3(VO4)2粉末对铀(VI)的去除性能, 并揭示其去除铀(VI)的机理。结果表明: 在pH 6、吸附时间2 h、吸附剂质量浓度0.1 g·L-1、初始铀(VI)质量浓度120 mg·L-1、吸附温度308 K条件下, Ca3(VO4)2粉末对铀(VI)具有较高的吸附容量(1179.92 mg·g-1)和去除率(98.33%); Ca3(VO4)2粉末对铀(VI)的去除机理为溶解和矿化, 最终生成准钒钙铀矿(Ca(UO2)2(VO4)2·3H2O)。在含有Zn2+、Cr3+、Cu2+、Ni2+、Co2+和Ba2+六种共存离子的水溶液中, Ca3(VO4)2粉末对铀(VI)仍具有较高的吸附容量和去除率, 且Ca3(VO4)2粉末能够将铀(VI)质量浓度从121.49 mg·L-1降低至0.1 mg·L-1, 该值低于相关国家排放标准(GB 23727-2020)。本研究制备的Ca3(VO4)2粉末作为一种处理含铀(VI)废水的吸附材料具有较好的应用前景。
中图分类号:
王红琴, 邓浩, 梁华, 田强, 晏敏皓, 黄毅. 正钒酸钙去除铀(VI)的性能与机理[J]. 无机材料学报, 2025, 40(11): 1268-1276.
WANG Hongqin, DENG Hao, LIANG Hua, TIAN Qiang, YAN Minhao, HUANG Yi. Properties and Mechanism of U(VI) Removal by Calcium Orthovanadate[J]. Journal of Inorganic Materials, 2025, 40(11): 1268-1276.
| Metal ion | ρ0 /(mg·L-1) | Metal ion | ρ0 /(mg·L-1) |
|---|---|---|---|
| Zn2+ | 648.63 | Co2+ | 67.12 |
| Cr3+ | 101.22 | Ba2+ | 64.22 |
| Cu2+ | 72.55 | U(VI) | 121.49 |
| Ni2+ | 133.04 |
表1 共存离子溶液中各金属离子的初始质量浓度
Table 1 Initial mass concentrations of several metal ions in coexisting ionic solution
| Metal ion | ρ0 /(mg·L-1) | Metal ion | ρ0 /(mg·L-1) |
|---|---|---|---|
| Zn2+ | 648.63 | Co2+ | 67.12 |
| Cr3+ | 101.22 | Ba2+ | 64.22 |
| Cu2+ | 72.55 | U(VI) | 121.49 |
| Ni2+ | 133.04 |
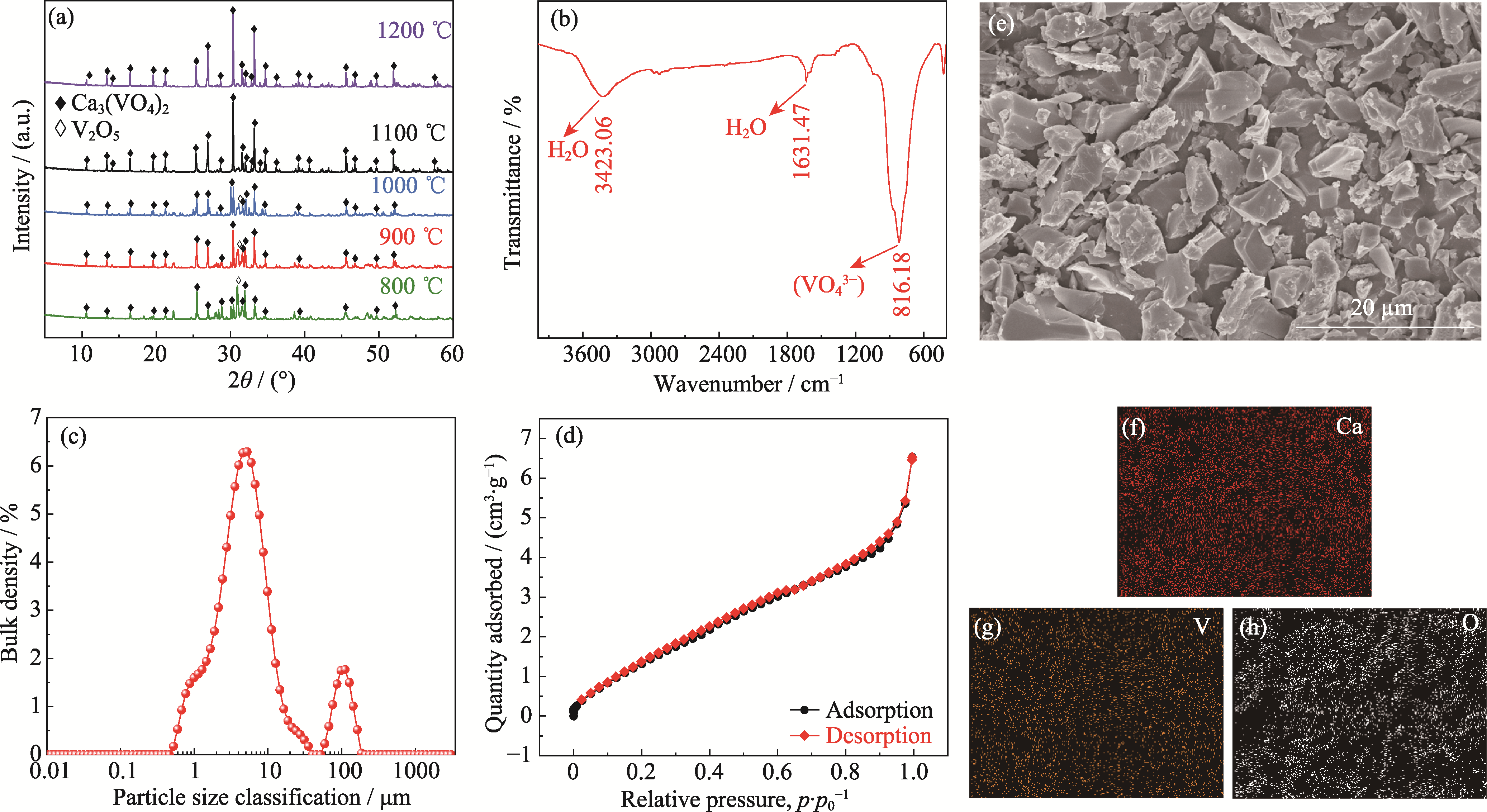
图1 Ca3(VO4)2粉末的表征结果
Fig. 1 Characterization results of Ca3(VO4)2 powder (a) XRD patterns of Ca3(VO4)2 prepared at different temperatures for 4 h; (b-h) FT-IR spectrum (b), particle size distribution (c), nitrogen adsorption-desorption curves (d), SEM image (e) and EDS mappings (f-h) of Ca3(VO4)2 prepared at 1100 ℃ for 4 h
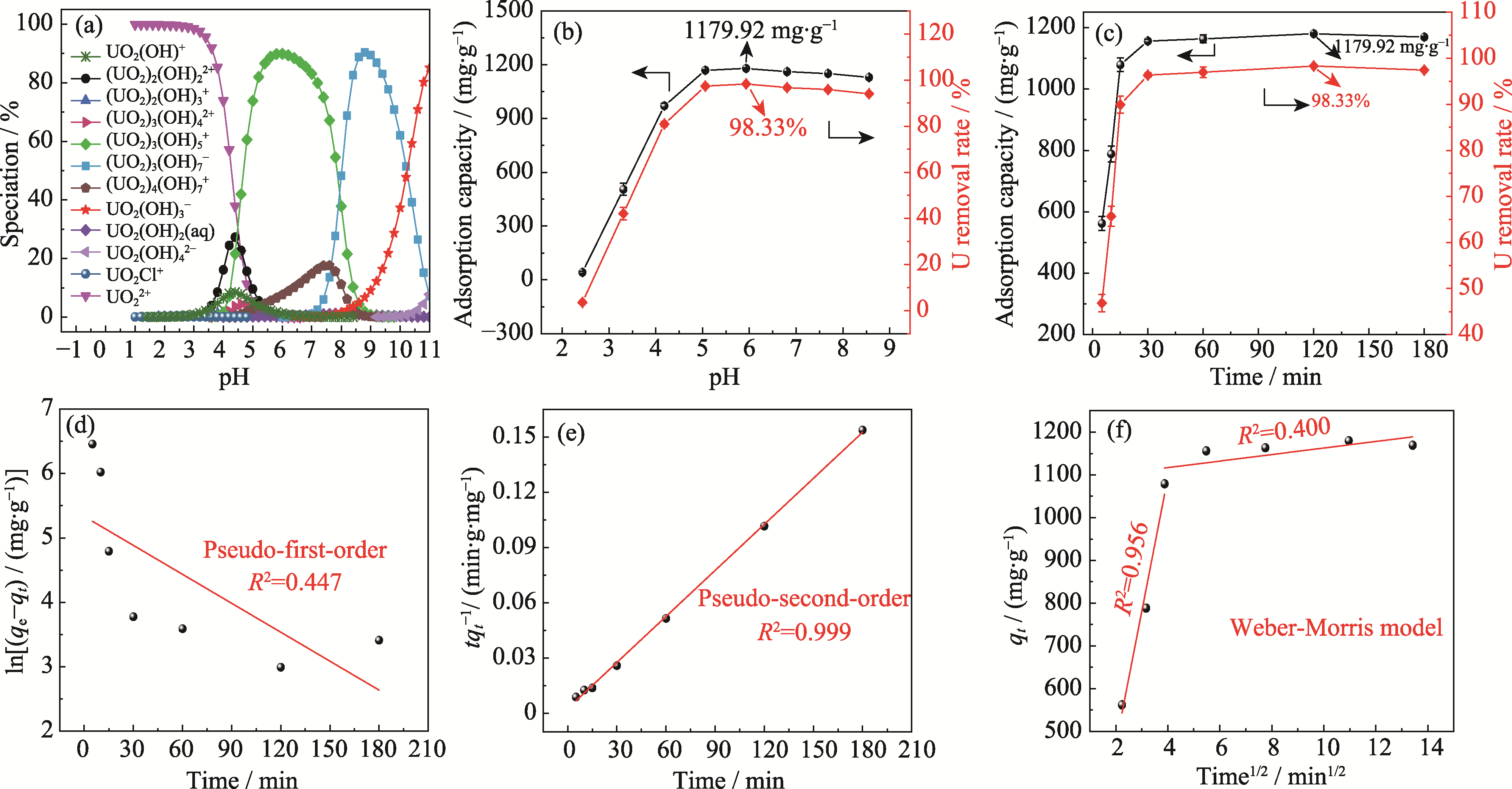
图2 pH、吸附时间对Ca3(VO4)2去除铀(VI)的影响
Fig. 2 Effect of pH and adsorption time on removal of U(VI) by Ca3(VO4)2 (a) Species distribution of U(VI) with different pH (U(VI) at mass concentration of 120 mg·L-1 under 308 K); (b, c) Adsorption capacity and removal rate of U(VI) with different pH (b) and durations (c); (d-f) Pseudo-first-order (d), pseudo-second-order (e) and Weber-Morris (f) models fitting (temperature at 308 K, initial U(VI) mass concentration at 120 mg·L-1, adsorbent dosage at 0.1 g·L-1)
| Material | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| k1/min-1 | R2 | k2/(g·mg-1·min-1) | R2 | |
| Ca3(VO4)2 | 0.01 | 0.447 | 2.62×10-4 | 0.999 |
表2 准一级和准二级动力学模型的拟合参数
Table 2 Fitting parameters of pseudo-first-order and pseudo-second-order kinetic models
| Material | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| k1/min-1 | R2 | k2/(g·mg-1·min-1) | R2 | |
| Ca3(VO4)2 | 0.01 | 0.447 | 2.62×10-4 | 0.999 |
| Material | Weber-Morris | |||
|---|---|---|---|---|
| kp,1/(mg·g-1·min-1/2) | R12 | kp,2/(mg·g-1·min-1/2) | R22 | |
| Ca3(VO4)2 | 312.25 | 0.956 | 7.65 | 0.400 |
表3 Weber-Morris动力学模型的拟合参数
Table 3 Fitting parameters of Weber-Morris dynamic model
| Material | Weber-Morris | |||
|---|---|---|---|---|
| kp,1/(mg·g-1·min-1/2) | R12 | kp,2/(mg·g-1·min-1/2) | R22 | |
| Ca3(VO4)2 | 312.25 | 0.956 | 7.65 | 0.400 |
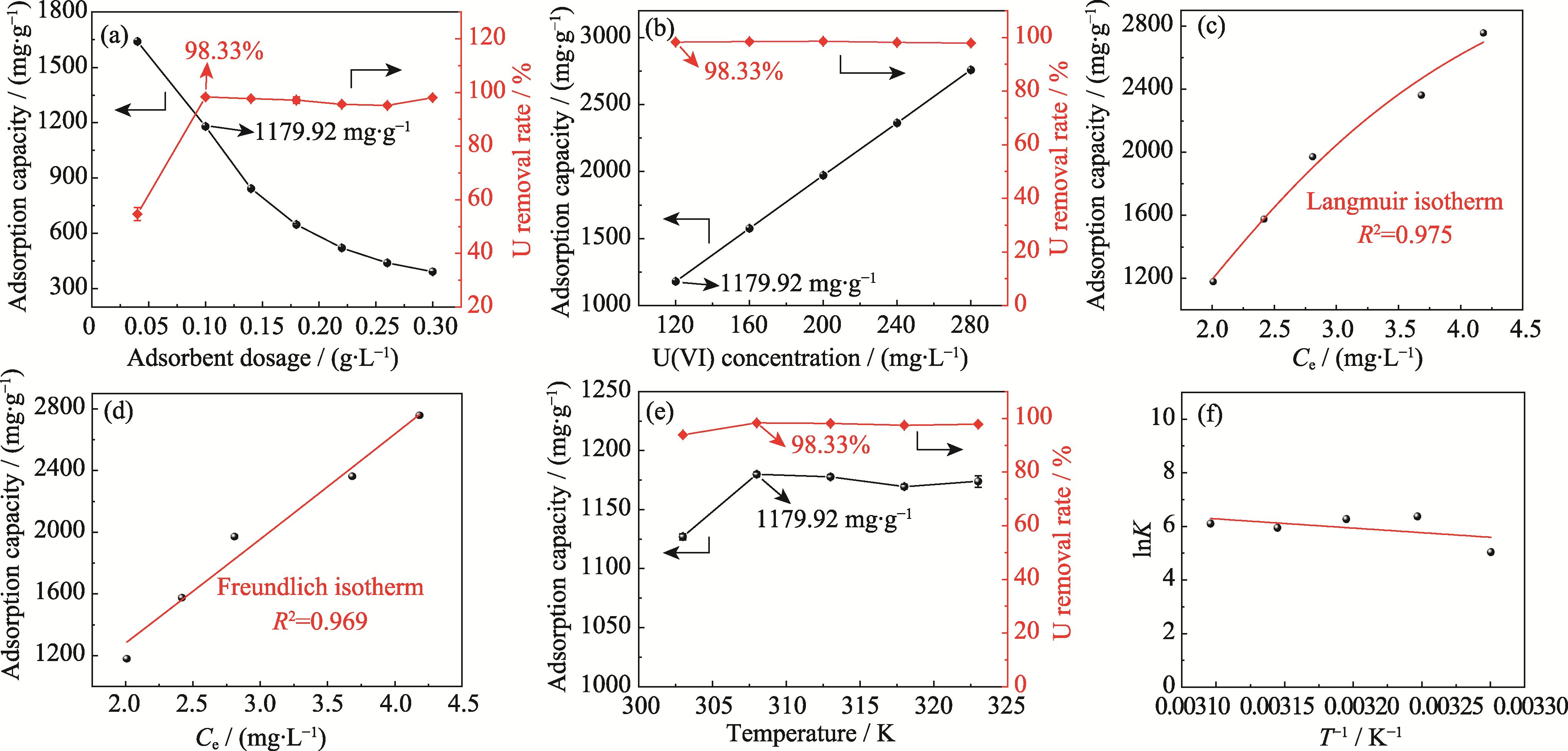
图3 吸附剂质量浓度、初始铀(VI)质量浓度和温度对Ca3(VO4)2去除铀(VI)的影响
Fig. 3 Effects of adsorbent dosage, initial U(VI) mass concentration, and temperature on removal of U(VI) by Ca3(VO4)2 (a, b) Adsorption capacity and removal rate of U(VI) with different adsorbent dosages (a) and initial U(VI) mass concentrations (b);(c, d) Langmuir (c) and Freundlich (d) isothermal adsorption models fitting; (e) Adsorption capacity and removal rate of U(VI) at different temperatures; (f) Relationship between lnK and 1/T (temperature at 308 K, pH 6, adsorption time at 2 h)
| Material | Freundlich | Langmuir | ||
|---|---|---|---|---|
| kF/(mg·g-1)·(mg·L-1)-1/n | R2 | kL/(L·mg-1) | R2 | |
| Ca3(VO4)2 | 623.24 | 0.969 | 0.09 | 0.975 |
表4 Freundlich和Langmuir等温吸附模型的拟合参数
Table 4 Fitting parameters of Freundlich and Langmuir isothermal adsorption models
| Material | Freundlich | Langmuir | ||
|---|---|---|---|---|
| kF/(mg·g-1)·(mg·L-1)-1/n | R2 | kL/(L·mg-1) | R2 | |
| Ca3(VO4)2 | 623.24 | 0.969 | 0.09 | 0.975 |
| Material | ∆H/(kJ·mol-1) | ∆S/(J·mol-1·K-1) | ∆G/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Ca3(VO4)2 | 28.55 | 140.74 | -14.09 | -14.79 | -15.50 | -16.20 | -16.91 |
表5 热力学拟合参数
Table 5 Thermodynamic fitting parameters
| Material | ∆H/(kJ·mol-1) | ∆S/(J·mol-1·K-1) | ∆G/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Ca3(VO4)2 | 28.55 | 140.74 | -14.09 | -14.79 | -15.50 | -16.20 | -16.91 |
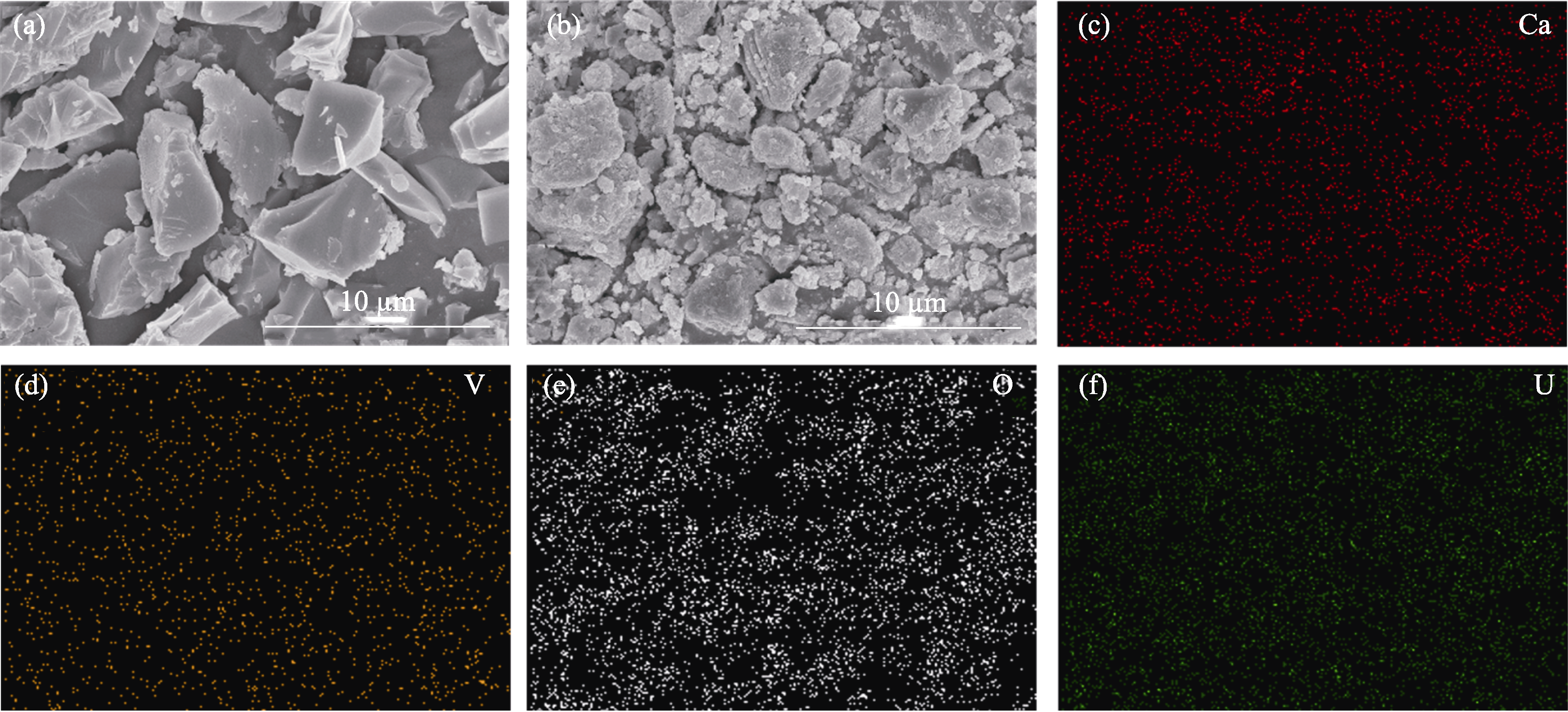
图4 吸附铀(VI)前后Ca3(VO4)2的SEM照片和表面元素分布
Fig. 4 SEM images and surface element distributions of Ca3(VO4)2 before and after adsorption of U(VI) (a, b) SEM images of Ca3(VO4)2 before (a) and after (b) adsorption of U(VI); (c-f) EDS mappings of Ca3(VO4)2 after adsorption of U(VI)
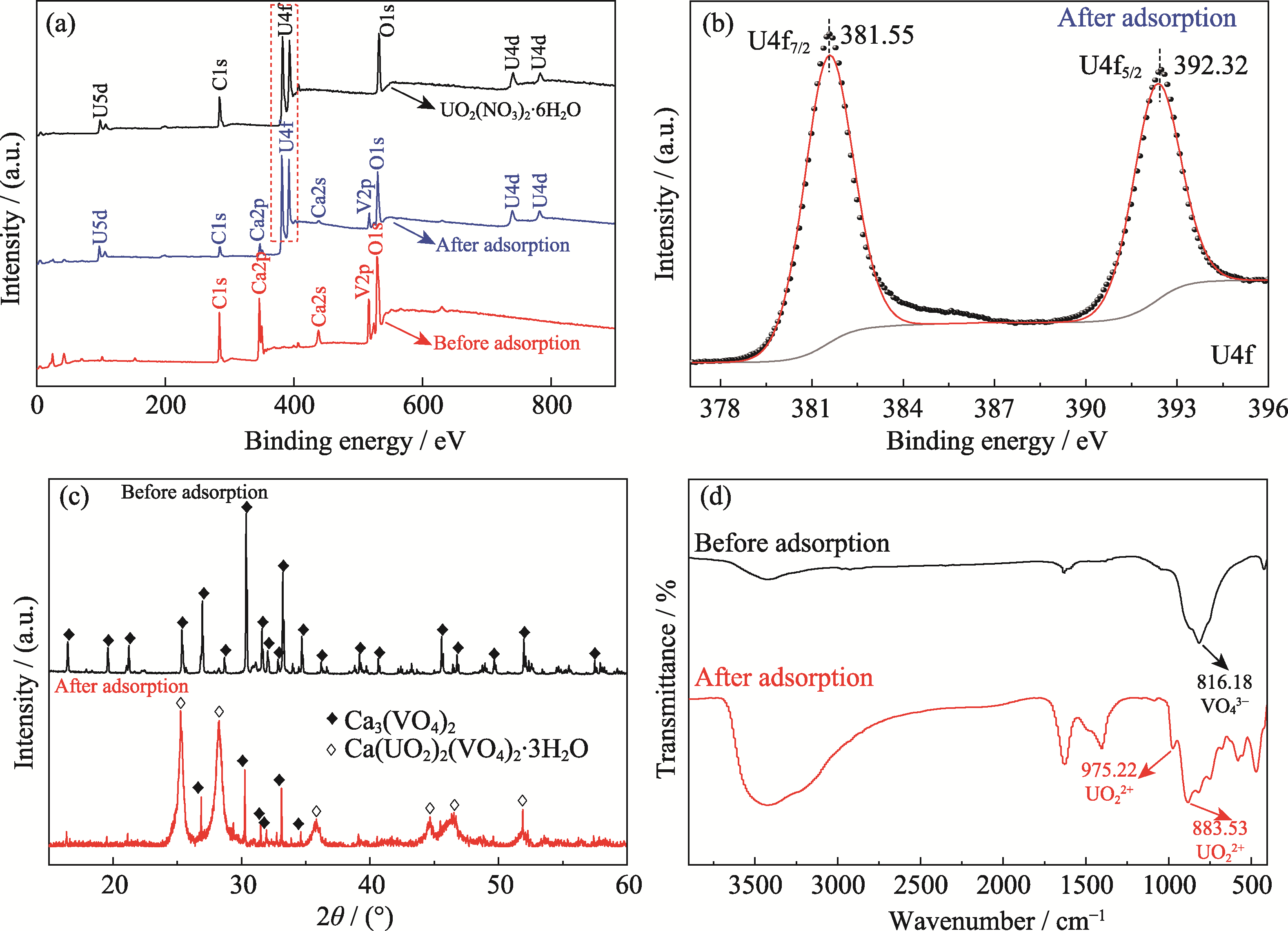
图5 吸附铀(VI)前后Ca3(VO4)2的表征结果
Fig. 5 Characterization results of Ca3(VO4)2 before and after adsorption of U(VI) (a) XPS spectra of Ca3(VO4)2 and UO2(NO3)2·6H2O; (b) U4f XPS spectrum of Ca3(VO4)2 after adsorption of U(VI); (c, d) XRD patterns (c) and FT-IR spectra (d) of Ca3(VO4)2
| Metal ion | ρ0/(mg·L-1) | Metal ion | ρ0/(mg·L-1) |
|---|---|---|---|
| Zn2+ | 588.55 | Co2+ | 63.66 |
| Cr3+ | 0.01 | Ba2+ | 60.51 |
| Cu2+ | 0.90 | U(VI) | 0.10 |
| Ni2+ | 126.60 |
表6 吸附后共存离子溶液中各金属离子的质量浓度
Table 6 Mass concentrations of several metal ions in coexisting ionic solution after adsorption
| Metal ion | ρ0/(mg·L-1) | Metal ion | ρ0/(mg·L-1) |
|---|---|---|---|
| Zn2+ | 588.55 | Co2+ | 63.66 |
| Cr3+ | 0.01 | Ba2+ | 60.51 |
| Cu2+ | 0.90 | U(VI) | 0.10 |
| Ni2+ | 126.60 |
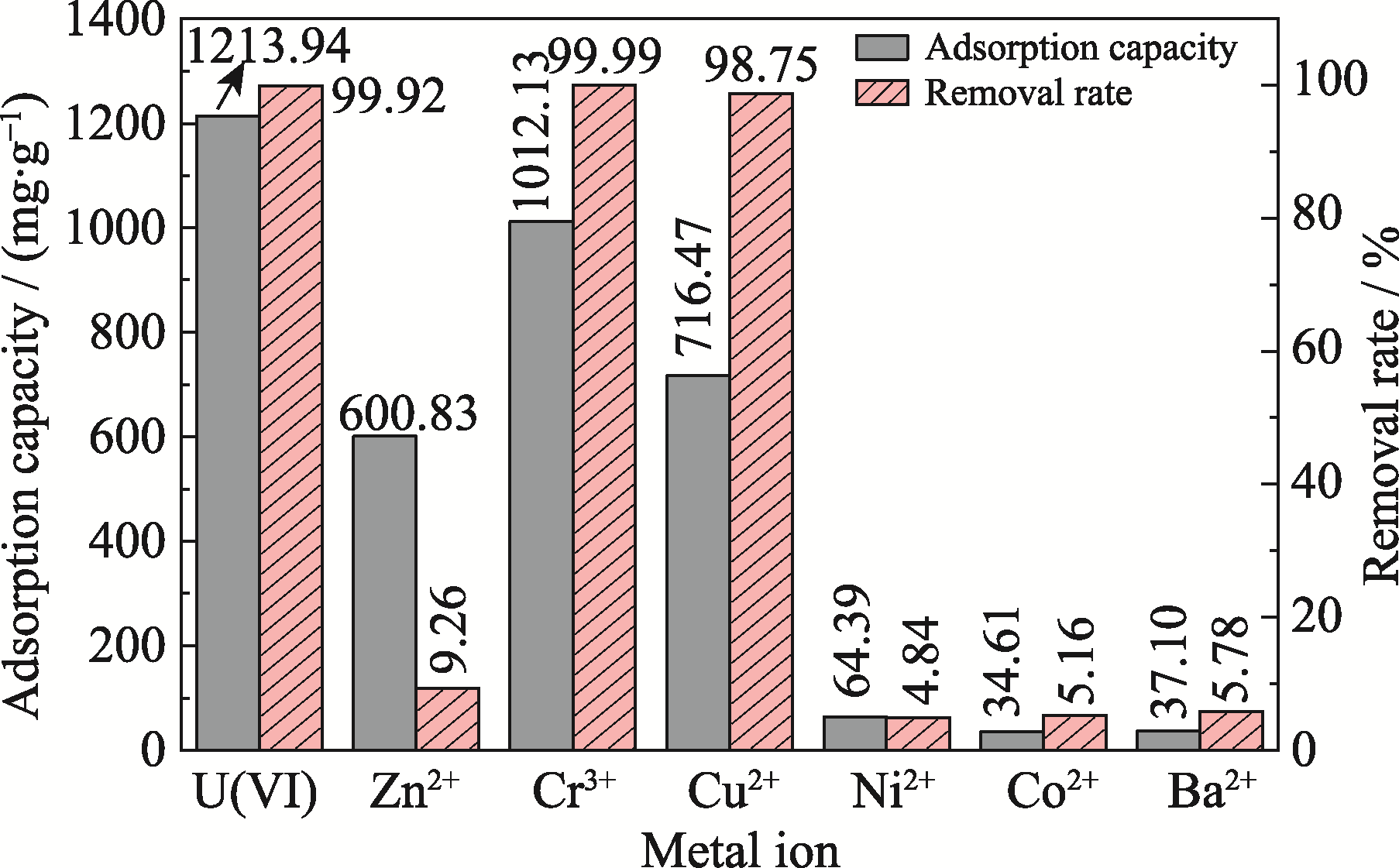
图6 共存离子溶液中Ca3(VO4)2对各金属离子的吸附容量和去除率
Fig. 6 Adsorption capacity and removal rate of Ca3(VO4)2 for several metal ions in coexisting ionic solution pH 6; Adsorption time: 2 h; Adsorbent dosage: 0.1 g·L-1; Temperature: 308 K
| Metal ion | Kd/(L·g-1) | k | Metal ion | Kd/(L·g-1) | k |
|---|---|---|---|---|---|
| U(VI) | 12039.43 | 1.00 | Ni2+ | 0.51 | 23673.64 |
| Zn2+ | 1.02 | 11793.32 | Co2+ | 0.54 | 22142.30 |
| Cr3+ | 168687.73 | 0.07 | Ba2+ | 0.61 | 19636.28 |
| Cu2+ | 791.97 | 15.20 |
表7 共存离子溶液中Ca3(VO4)2对各金属离子的选择性吸附参数
Table 7 Selective adsorption parameters of Ca3(VO4)2 for several metal ions in coexisting ionic solution
| Metal ion | Kd/(L·g-1) | k | Metal ion | Kd/(L·g-1) | k |
|---|---|---|---|---|---|
| U(VI) | 12039.43 | 1.00 | Ni2+ | 0.51 | 23673.64 |
| Zn2+ | 1.02 | 11793.32 | Co2+ | 0.54 | 22142.30 |
| Cr3+ | 168687.73 | 0.07 | Ba2+ | 0.61 | 19636.28 |
| Cu2+ | 791.97 | 15.20 |
| Material | Adsorption capacity/(mg·g-1) | pH | Ref. |
|---|---|---|---|
| Mg2CO3(OH)2 | 370.00 | 5.0 | [ |
| Crab carapace | 1.38 | 8.0 | [ |
| PG/SFA | 84.60 | 6.0 | [ |
| HAP | 130.08 | 5.0 | [ |
| Kaolinite | 9.17 | 4.5 | [ |
| ZSM-12 zeolite | 12.00 | 3.0 | [ |
| H11Al2V6O23.2 | 917.00 | 5.0 | [ |
| EuVO4 | 276.16 | 4.5 | [ |
| Ca3(VO4)2 | 1179.92 | 6.0 | This study |
表8 Ca3(VO4)2与其他吸附材料对铀(VI)的吸附容量[11-12,15,33-37]
Table 8 Adsorption capacity of Ca3(VO4)2 and other adsorbents for uranium (VI) removal[11-12,15,33-37]
| Material | Adsorption capacity/(mg·g-1) | pH | Ref. |
|---|---|---|---|
| Mg2CO3(OH)2 | 370.00 | 5.0 | [ |
| Crab carapace | 1.38 | 8.0 | [ |
| PG/SFA | 84.60 | 6.0 | [ |
| HAP | 130.08 | 5.0 | [ |
| Kaolinite | 9.17 | 4.5 | [ |
| ZSM-12 zeolite | 12.00 | 3.0 | [ |
| H11Al2V6O23.2 | 917.00 | 5.0 | [ |
| EuVO4 | 276.16 | 4.5 | [ |
| Ca3(VO4)2 | 1179.92 | 6.0 | This study |
| [1] |
XIE Y H, YU L, CHEN L, et al. Recent progress of radionuclides separation by porous materials. Science China Chemistry, 2024, 67(11): 3515.
DOI |
| [2] |
ZHANG X Y, YAN M J, CHEN P, et al. Emerging MOFs, COFs, and their derivatives for energy and environmental applications. The Innovation, 2025, 6(2): 100778.
DOI URL |
| [3] | 谢永波, 曾涛涛, 王国华, 等. 铀矿山生态环境修复. 北京: 科学出版社, 2021. |
| [4] |
KHARE D, ACHARYA C. Uranium biomineralization by immobilized Chryseobacterium sp. strain PMSZPI cells for efficient uranium removal. Journal of Hazardous Materials, 2024, 465: 133503.
DOI URL |
| [5] |
MEZA I, HUA H, GAGNON K, et al. Removal of aqueous uranyl and arsenate mixtures after reaction with limestone, PO43-, and Ca2+. Environmental Science & Technology, 2023, 57(49): 20881.
DOI URL |
| [6] |
YU Y, LIU J Y, CHEN S S, et al. Bioinspired electrostatic layer-by-layer assembly membranes constructed based on mild strategy for uranium extraction from seawater. Chemical Engineering Journal, 2024, 486: 149783.
DOI URL |
| [7] |
LI X, LIU Z R, HUANG M. Purification of uranium-containing wastewater by adsorption: a review of research on resin materials. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(7): 3043.
DOI |
| [8] |
YU C X, JIANG W, LEI M, et al. Fabrication of carboxylate- functionalized 2D MOF nanosheet with caged cavity for efficient and selective extraction of uranium from aqueous solution. Small, 2024, 20(23): 2308910.
DOI URL |
| [9] |
ZHONG X, TAN Y B, WU S Y, et al. Efficient and rapid capture of uranium(VI) in wastewater via multi-amine modified β-cyclodextrin porous polymer. Chinese Journal of Chemical Engineering, 2024, 68: 144.
DOI URL |
| [10] | 王浩, 陈枫, 柯倩, 等. 氮化硼负载磷钨酸铁对U(VI)的吸附及其机理研究. 中国科学: 化学, 2019, 49(1): 123. |
| [11] |
FENG S W, FENG L J, WANG M, et al. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chemical Engineering Journal, 2022, 430: 133038.
DOI URL |
| [12] | 夏雪, 周磊, 杨国辉, 等. 磷石膏/固硫灰渣复合材料对铀(VI)的吸附行为及机理研究. 金属矿山, 2023(11): 81. |
| [13] |
WU T N, WANG M B, ZHONG T T, et al. Behavior of uranium immobilization with hydroxyapatite and dissolution stability of the immobilization product. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(3): 647.
DOI |
| [14] |
DAN H, CHEN L Y, XIAN Q, et al. Tailored synthesis of SBA-15 rods using different types of acids and its application in adsorption of uranium. Separation and Purification Technology, 2019, 210: 491.
DOI URL |
| [15] |
LIN Y L, LIU Y H, ZHANG S, et al. Electrochemical synthesis of EuVO4 for the adsorption of U(VI): performance and mechanism. Chemosphere, 2021, 273: 128569.
DOI URL |
| [16] | LAUF R J. Mineralogy of uranium and thorium. Atglen: Schiffer Publishing, 2016. |
| [17] |
BANERJEE C, DUDWADKAR N, TRIPATHI S C, et al. Nano-cerium vanadate: a novel inorganic ion exchanger for removal of americium and uranium from simulated aqueous nuclear waste. Journal of Hazardous Materials, 2014, 280: 63.
DOI PMID |
| [18] |
KARYAKIN N V, CHERNORUKOV N G, SULEIMANOV E V, et al. Thermodynamics of alkali metal uranovanadates. Russian Journal of General Chemistry, 2001, 71(9): 1333.
DOI |
| [19] |
KARYAKIN N V, CHERNORUKOV N G, SULEIMANOV E V, et al. Chemical thermodynamics of alkaline-earth metal uranovanadates. Radiochemistry, 2003, 45(5): 457.
DOI |
| [20] |
杜浪, 李玉香, 马雪, 等. 偶氮胂Ⅲ分光光度法测定微量铀. 冶金分析, 2015, 35(1): 68.
DOI |
| [21] | 门倩, 唐振平, 刘江, 等. 湘南某铀矿山周边水体放射性金属及重金属污染特征. 湖北农业科学, 2019, 58(15): 39. |
| [22] |
YUE H R, XUE X X. Evolution of generated calcium vanadates at different locations in the vicinity of the V2O5/CaO interface with annealing parameters. Metallurgical and Materials Transactions B, 2020, 51(5): 2358.
DOI |
| [23] |
PARHI P, MANIVANNAN V, KOHLI S, et al. Synthesis and characterization of M3V2O8 (M=Ca, Sr and Ba) by a solid-state metathesis approach. Bulletin of Materials Science, 2008, 31(6): 885.
DOI URL |
| [24] |
CHEN J L, YANG X, WANG L Y, et al. Graphene oxide wrapped Cu-MOF as an efficient adsorbent for uranium extraction from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 2024, 333(1): 263.
DOI |
| [25] |
LIAO J, HE X S, ZHANG Y, et al. The construction of magnetic hydroxyapatite-functionalized pig manure-derived biochar for the efficient uranium separation. Chemical Engineering Journal, 2023, 457: 141367.
DOI URL |
| [26] |
LIU Y F, NI S, WANG W J, et al. Facile and scalable synthesis of functionalized hierarchical porous polymers for efficient uranium adsorption. Water Research, 2024, 257: 121683.
DOI URL |
| [27] |
GUO X, FENG Y R, MA L, et al. Phosphoryl functionalized mesoporous silica for uranium adsorption. Applied Surface Science, 2017, 402: 53.
DOI URL |
| [28] |
PENG T Q, WANG Y F, XU Y F, et al. Synthesis, characterization and uranium (VI) adsorption mechanism of novel adsorption material poly(tetraethylenepentamine-trimesoyl chloride). Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(2): 409.
DOI |
| [29] |
WANG Q, WANG Y, WU Y Q, et al. Salicylhydroxamic acid intercalated layered double hydroxide for efficient uranium uptake from seawater. Journal of Environmental Chemical Engineering, 2025, 13(1): 115055.
DOI URL |
| [30] |
CHERNORUKOV N G, NIPRUK O V, KNYAZEV A V, et al. Uranyl orthovanadate of composition (UO2)3(VO4)2·4H2O: synthesis and characterization. Russian Journal of Inorganic Chemistry, 2013, 58(5): 506.
DOI URL |
| [31] | MOLLICK S, SAURABH S, MORE Y D, et al. Benchmark uranium extraction from seawater using an ionic macroporous metal-organic framework. Energy & Environmental Science, 2022, 15(8): 3462. |
| [32] |
赵凯鑫, 高琼, 田强, 等. 磷酸三钙去除U(VI)的性能与机理研究. 核化学与放射化学, 2023, 45(4): 364.
DOI |
| [33] |
ZHANG L, JING X Y, LI R M, et al. Magnesium carbonate basic coating on cotton cloth as a novel adsorbent for the removal of uranium. RSC Advances, 2015, 5(30): 23144.
DOI URL |
| [34] |
LI L X, ZHOU Z K, WANG G H, et al. Adsorption performance and its mechanism of uranium using rod-like hydroxyapatite by one- step hydrothermal method. Physica Scripta, 2024, 99(8): 085944.
DOI |
| [35] |
ISSA R A M, AMARI A O E, ALHANASH H B, et al. Removal of uranium (VI) ion from aqueous solution using kaolinite. Kuwait Journal of Science, 2023, 50(4): 609.
DOI URL |
| [36] |
KHEMAISSIA S, ABAIDIA R, HOUHOUNE F, et al. Synthesis and characterization of ZSM-12 zeolite for uranium(VI) adsorption: isotherm, kinetic, and thermodynamic investigations. Comptes Rendus Chimie, 2025, 28: 79.
DOI URL |
| [37] |
LUO J Q, CHEN J L, CHEN J, et al. Aluminum vanadate microspheres is a simple but effective material for uranium extraction: performance and mechanism. Journal of Solid State Chemistry, 2022, 312: 123237.
DOI URL |
| [1] | 蔡豪, 汪琦航, 邹朝勇. 镁离子调控无定形碳酸钙制备一水碳酸钙结晶过程[J]. 无机材料学报, 2024, 39(11): 1275-1282. |
| [2] | 吴锐, 张敏慧, 金成韵, 林健, 王德平. 光热核壳TiN@硼硅酸盐生物玻璃纳米颗粒的降解和矿化性能[J]. 无机材料学报, 2023, 38(6): 708-716. |
| [3] | 马磊, 黄毅, 邓浩, 银航, 田强, 晏敏皓. 氟磷灰石对酸性水溶液中铀(VI)的去除研究[J]. 无机材料学报, 2022, 37(4): 395-403. |
| [4] | 符明富, 杨雯, 李佳保, 邓书康, 周启航, 冯小波, 杨培志. 化学气相运输法制备正交黑磷[J]. 无机材料学报, 2022, 37(10): 1102-1108. |
| [5] | 朱子旻, 张敏慧, 张轩宇, 姚爱华, 林健, 王德平. 硼硅酸盐生物活性玻璃在直流电场下的体外矿化性能[J]. 无机材料学报, 2021, 36(9): 1006-1012. |
| [6] | 余祥坤, 刘坤, 李志鹏, 赵雨露, 沈锦优, 茆平, 孙爱武, 蒋金龙. 铜/凹凸棒石复合材料高效吸附放射性碘离子性能[J]. 无机材料学报, 2021, 36(8): 856-864. |
| [7] | 许宏一, 翟东, 曹琬婷, 陈振华, 钱文昊, 陈蕾. Li2Ca2Si2O7生物陶瓷的矿化活性研究[J]. 无机材料学报, 2021, 36(7): 753-760. |
| [8] | 刘继涛, 钏定泽, 杨泽斌, 陈希亮, 颜廷亭, 陈庆华. 氨基酸/羟基磷灰石复合材料应用于酸蚀牛牙釉质体外再矿化[J]. 无机材料学报, 2019, 34(11): 1222-1230. |
| [9] | 董志红, 聂志萍, 周长春. 自然唾液中介孔生物活性玻璃诱导牙釉质仿生再矿化研究[J]. 无机材料学报, 2016, 31(1): 88-94. |
| [10] | 许金妹, 刘新玲, 高彦峰. 多巴胺辅助牙本质沉积羟基磷灰石研究[J]. 无机材料学报, 2016, 31(1): 95-99. |
| [11] | 马玉菲, 乔 莉, 冯庆玲. 淡水珍珠的生物矿化机理研究进展[J]. 无机材料学报, 2013, 28(1): 109-116. |
| [12] | 朱云荣, 陈玉云, 许国华, 叶晓健, 钟 健, 何丹农. 丝素蛋白含量对纳米羟基磷灰石仿生矿化和体外细胞相容性的影响[J]. 无机材料学报, 2012, 27(8): 883-886. |
| [13] | 程 新, 李延报, 陆春华, 李东旭, 许仲梓. 预处理对构建丝素蛋白纤维/磷灰石仿生骨修复材料的影响[J]. 无机材料学报, 2011, 26(1): 43-48. |
| [14] | 杨 锦, 李君君, 袁欢欣, 欧阳健明. 氨羧钾调控草酸钙晶体生长及其与分子结构的关系[J]. 无机材料学报, 2010, 25(11): 1185-1190. |
| [15] | 熊浩洋,胡彬彬,薛中会,蔡 莉,戴树玺,杜祖亮. 牛血清蛋白单层分子膜诱导生长PbS晶体[J]. 无机材料学报, 2010, 25(1): 63-67. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||