无机材料学报 ›› 2023, Vol. 38 ›› Issue (7): 750-762.DOI: 10.15541/jim20220580 CSTR: 32189.14.10.15541/jim20220580
所属专题: 【生物材料】骨骼与齿类组织修复(202506)
赵睿1,2( ), 毛飞1, 钱晖1(
), 毛飞1, 钱晖1( ), 杨晓2, 朱向东2, 张兴栋2
), 杨晓2, 朱向东2, 张兴栋2
收稿日期:2022-09-30
修回日期:2022-10-27
出版日期:2023-03-06
网络出版日期:2023-03-06
通讯作者:
钱 晖, 教授. E-mail: 1000007341@ujs.edu.cn作者简介:赵 睿(1995-), 女, 博士, 讲师. E-mail: 1000005729@ujs.edu.cn
基金资助:
ZHAO Rui1,2( ), MAO Fei1, QIAN Hui1(
), MAO Fei1, QIAN Hui1( ), YANG Xiao2, ZHU Xiangdong2, ZHANG Xingdong2
), YANG Xiao2, ZHU Xiangdong2, ZHANG Xingdong2
Received:2022-09-30
Revised:2022-10-27
Published:2023-03-06
Online:2023-03-06
Contact:
QIAN Hui, professor. E-mail: 1000007341@ujs.edu.cnAbout author:ZHAO Rui (1995-), female, PhD, lecturer. E-mail: 1000005729@ujs.edu.cn
Supported by:摘要:
天然骨组织由有机纳米材料胶原纤维和无机纳米材料羟基磷灰石组成, 具有独特的微纳米结构以及传统人工合成材料无法比拟的生物功能和力学性能优势。在组织工程和再生医学的研究中, 模拟天然骨组织层次特征的微纳米结构生物材料是研究热点之一。近年来, 研究人员发现微纳米结构生物材料能有效调节细胞增殖、分化和迁移, 促进细胞成骨分化, 进而促进体内骨组织再生。本文综述了利用天然骨组织层次特征指导材料分层设计的研究进展以及微纳米结构生物材料的细胞相互作用特性和在骨组织工程中的应用, 以期为生物材料的设计提供新思路。
中图分类号:
赵睿, 毛飞, 钱晖, 杨晓, 朱向东, 张兴栋. 微纳米结构生物材料在骨组织再生修复中的研究进展[J]. 无机材料学报, 2023, 38(7): 750-762.
ZHAO Rui, MAO Fei, QIAN Hui, YANG Xiao, ZHU Xiangdong, ZHANG Xingdong. Micro-/Nano-structured Biomaterials for Bone Regeneration: New Progress[J]. Journal of Inorganic Materials, 2023, 38(7): 750-762.
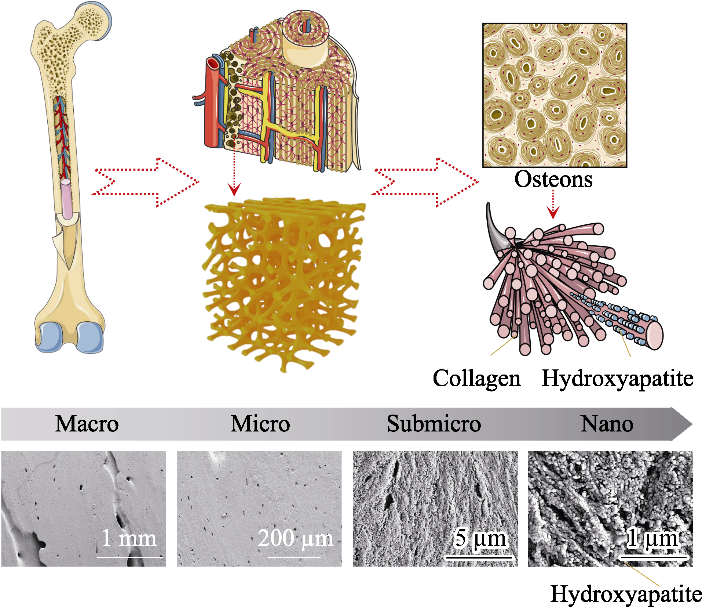
图1 天然骨组织的层次结构模式图和人股骨骨干部皮质骨扫描电镜照片[1,11]
Fig. 1 Schematic diagram of bone hierarchical structural organization (up part) and scanning electron microscope images (bottom part) of the cortical bone specimens located at human femoral diaphysis[1,11] In bone tissue, macroscale arrangements involve both compact/ cortical bone at the surface and spongy/trabecular bone in the interior. Compact bone is composed of osteons and Haversian canals, which surrounded by blood vessels. Osteons have a lamellar structure, with individual lamella consisting of fibers arranged in geometrical patterns. The fibers comprise several mineralized collagen fibrils, composed of collagen protein molecules formed from three chains of amino acids and nanocrystals of hydroxyapatite, and linked by an organic phase to form fibril arrays
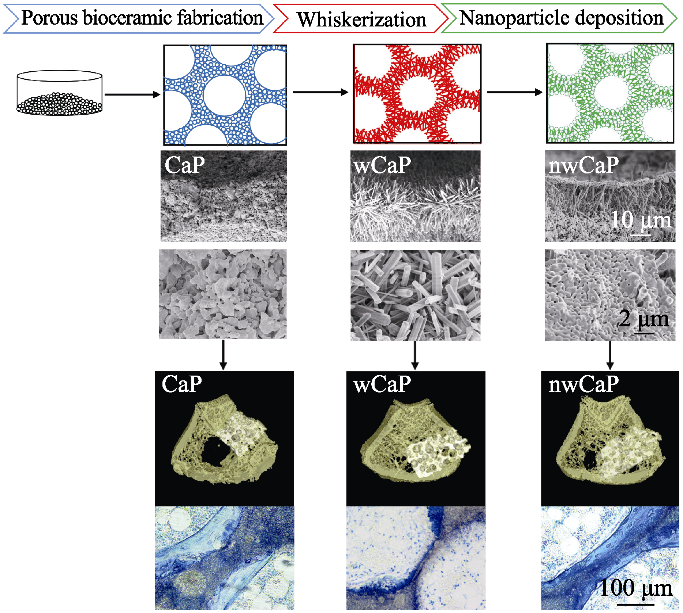
图2 不同表面形貌的传统磷酸钙(CaP)、晶须化磷酸钙(wCaP)和微纳米结构磷酸钙(nwCaP)生物陶瓷制备流程图和成骨效果[33]
Fig. 2 Schematic diagram of preparation process and bone forming ability of traditional calcium phosphate (CaP), whiskered calcium phosphate (wCaP) and micro-/nano-structured calcium phosphate (nwCaP) bioceramics with different surface morphologies[33]

图3 微纳米结构磷酸钙(nwCaP)生物陶瓷诱导成骨所涉及的分子机制研究[33]
Fig. 3 Illustration of the possible molecular mechanism involved in nwCaP bioceramics induced osteogenic effect[33] (a) Photos of Alizarin Red S and von Kossa stainings; (b) Cluster analysis of genes and quantitative qRT-PCR analysis expressions; (c) Osteogenesis-related gene expression; (d) Representative western blot analysis; OVX: Ovariectomized

图4 免疫荧光染色评估微纳米结构羟基磷灰石生物陶瓷内部的血管生成[16]
Fig. 4 CD31 and EMCN staining of histological sections from the micro-/nano-structured hydroxyapatite bioceramic groups[16] Green fluorescence: CD31; Red fluorescence: EMCN; Blue fluorescence: Nucleus of the cells

图5 连续荧光标记评估动态骨形成[16]
Fig. 5 In vivo sequential fluorescence labeling of new bone formation inside porous nwHA bioceramics[16] (a) Observed patterns of new bone formation (yellow: tetracycline label; green: calcein label); (b) Two types of osteogenesis discovered inside the pore structure of nwHA bioceramics (green indicating CD 31, Red indicating EMCN, and blue indicating nucleus); (c) Comparison of mineral apposition rate (MAR) between different nwHA groups, with statistical analysis of the relationship between osteogenesis type and pore diameter of the bioceramics, and the relationship between osteogenesis type and MAR
| Material | Synthesis method | In vitro results | Animal model | In vivo results | Ref. |
|---|---|---|---|---|---|
| β-TCP scaffolds with micro/ nano surface topography | DLP printing and in situ growth crystal process | Promote osteogenic differentiation of stem cells | Rat skull defects | Improve the bone regeneration | [ |
| Micro/nano-scale titania fiber-like network on the surface of Ti implants | One-step alkaline treatment in NaOH solution | Facilitate osteogenic and angiogenic differentiation of BMSCs and endothelial cells; Suppress M1 macrophages and stimulate M2 phenotype | Rabbit femur defects | Induce ameliorative osseointegration | [ |
| MNBG/PLGA bi-layered membranes | Electrospinning | Promote osteogenesis | [ | ||
| Micro-nano rough Ti6Al4V | Acid etch process | Improve osteogenic differentiation of MSCs | [ | ||
| HA bioceramics with submicron- to nano- topographies | Sintering | Maintain the conformation of BMP-2, activate the osteogenic differentiation of BMSCs | Canine intramuscular implantation | Process excellent bone-like apatite forming ability and outstanding osteoinductivity | [ |
| HA with micro/nano hierarchical structures | Photolithography and hydrothermal techniques | Promote osteogenic differentiation of hBMSCs and angiogenic acticvity of HUVECs | [ | ||
| β-TCP/CaSiO3 composite ceramics with micro/ nano-HAp the surface layer | 3D bioplotting and hydrothermal treatment | Upregulate the cellular differentiation of mBMSCs and gene expression of HUVECs | Ectopic subcutaneous implantation at the back of rats | Promote capillary formation and bone augmentation | [ |
| PEEK/CF/n-HA ternary biocomposite with micro/ nano-topographical surface | Oxygen plasma and sandblasting | Promote the proliferation and differentiation of MG-63 cells | Dog mandibles | Boost the osseointegration between implant and bone | [ |
| Micro/nano structural silicon nitride and PEKK composite | Femtosecond laser ablation | Promote osteogenic differentiation of rBMSCs; Exhibit a greater bacteriostatic activity | Rabbit femur cavity defect | Promote osseointegration and bone repair | [ |
| Silicate-based bioceramic with micro-nano surfaces and hollow channels | 3D printing and hydrothermal treatment | Facilitate the attachment and proliferation of BMSCs | Rabbit femur defects | Boost the newly bone formation | [ |
| PLLA/CS composite scaffold with micro/nano- fiber hierarchical structure | 3D printing and thermally induced phase separation technology | Promote cell adhesion and proliferation | [ |
表1 微纳米结构生物材料用于成骨研究的文献总结
Table 1 Summary of previous work on bone formation in the micro-/nano-structured biomaterials
| Material | Synthesis method | In vitro results | Animal model | In vivo results | Ref. |
|---|---|---|---|---|---|
| β-TCP scaffolds with micro/ nano surface topography | DLP printing and in situ growth crystal process | Promote osteogenic differentiation of stem cells | Rat skull defects | Improve the bone regeneration | [ |
| Micro/nano-scale titania fiber-like network on the surface of Ti implants | One-step alkaline treatment in NaOH solution | Facilitate osteogenic and angiogenic differentiation of BMSCs and endothelial cells; Suppress M1 macrophages and stimulate M2 phenotype | Rabbit femur defects | Induce ameliorative osseointegration | [ |
| MNBG/PLGA bi-layered membranes | Electrospinning | Promote osteogenesis | [ | ||
| Micro-nano rough Ti6Al4V | Acid etch process | Improve osteogenic differentiation of MSCs | [ | ||
| HA bioceramics with submicron- to nano- topographies | Sintering | Maintain the conformation of BMP-2, activate the osteogenic differentiation of BMSCs | Canine intramuscular implantation | Process excellent bone-like apatite forming ability and outstanding osteoinductivity | [ |
| HA with micro/nano hierarchical structures | Photolithography and hydrothermal techniques | Promote osteogenic differentiation of hBMSCs and angiogenic acticvity of HUVECs | [ | ||
| β-TCP/CaSiO3 composite ceramics with micro/ nano-HAp the surface layer | 3D bioplotting and hydrothermal treatment | Upregulate the cellular differentiation of mBMSCs and gene expression of HUVECs | Ectopic subcutaneous implantation at the back of rats | Promote capillary formation and bone augmentation | [ |
| PEEK/CF/n-HA ternary biocomposite with micro/ nano-topographical surface | Oxygen plasma and sandblasting | Promote the proliferation and differentiation of MG-63 cells | Dog mandibles | Boost the osseointegration between implant and bone | [ |
| Micro/nano structural silicon nitride and PEKK composite | Femtosecond laser ablation | Promote osteogenic differentiation of rBMSCs; Exhibit a greater bacteriostatic activity | Rabbit femur cavity defect | Promote osseointegration and bone repair | [ |
| Silicate-based bioceramic with micro-nano surfaces and hollow channels | 3D printing and hydrothermal treatment | Facilitate the attachment and proliferation of BMSCs | Rabbit femur defects | Boost the newly bone formation | [ |
| PLLA/CS composite scaffold with micro/nano- fiber hierarchical structure | 3D printing and thermally induced phase separation technology | Promote cell adhesion and proliferation | [ |
| [1] |
HOSEINPOUR V, SHARIATINIA Z. Applications of zeolitic imidazolate framework-8 (ZIF-8) in bone tissue engineering: a review. Tissue Cell, 2021, 72: 101588.
DOI URL |
| [2] | BUSCH A, JÄGER M, MAYER C, et al. Functionalization of synthetic bone substitutes. International Journal of Molecuilar Science, 2021, 22(9): 4412. |
| [3] |
HUANG X, CHEN Q, LUO W, et al. SATB2: a versatile transcriptional regulator of craniofacial and skeleton development, neurogenesis and tumorigenesis, and its applications in regenerative medicine. Genes Disease, 2022, 9(1): 95.
DOI URL |
| [4] | GOHIL S V, ADAMS D J, MAYE P, et al. Evaluation of rhBMP-2 and bone marrow derived stromal cell mediated bone regeneration using transgenic fluorescent protein reporter mice. Journal of Biomedical Materials Research A, 2014, 102(12): 4568. |
| [5] |
GRAYSON W L, BUNNELL B A, MARTIN E, et al. Stromal cells and stem cells in clinical bone regeneration. Nature Reviews: Endocrinology, 2015, 11(3): 140.
DOI |
| [6] |
SI L, WINZENBERG T M, JIANG Q, et al. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporosis International, 2015, 26(7): 1929.
DOI PMID |
| [7] |
AGARWAL R, GONZÁLEZ-GARCÍA C, TORSTRICK B, et al. Simple coating with fibronectin fragment enhances stainless steel screw osseointegration in healthy and osteoporotic rats. Biomaterials, 2015, 63: 137.
DOI PMID |
| [8] |
ARCOS D, BOCCACCINI A R, BOHNER M, et al. The relevance of biomaterials to the prevention and treatment of osteoporosis. opinion paper. Acta Biomaterialia, 2014, 10(5): 1793.
DOI PMID |
| [9] |
GIACOMINI D, TORRICELLI P, GENTILOMI G A, et al. Monocyclic β-lactams loaded on hydroxyapatite: new biomaterials with enhanced antibacterial activity against resistant strains. Scientific Reports, 2017, 7(1): 2712.
DOI |
| [10] | E L, LU R, SUN J, et al. Microenvironment influences on human umbilical cord mesenchymal stem cell-based bone regeneration. Stem Cells International, 2021, 2021: 4465022. |
| [11] |
DU Y, GUO J L, WANG J, et al. Hierarchically designed bone scaffolds: from internal cues to external stimuli. Biomaterials, 2019, 218: 119334.
DOI URL |
| [12] |
TANG Z, LI X, TAN Y, et al. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regenerative Biomaterials, 2018, 5(1): 43.
DOI PMID |
| [13] |
ZHOU P, WU J, XIA Y, et al. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. International Journal of Nanomedicine, 2018, 13: 4083.
DOI PMID |
| [14] | CUI F Z, MIKOS A G. Important topics in the future of tissue engineering:comments from the participants of the 5th International Conference on Tissue Engineering at Kos, Greece. Regenerative Biomaterials, 2014, 1(1): 103. |
| [15] | LIU H, ZHANG Z, SEONG TOH W, et al. Stem cells: microenvironment, micro/nanotechnology, and application. Stem Cells International, 2015, 2015: 398510. |
| [16] |
ZHAO R, SHANG T, YUAN B, et al. Osteoporotic bone recovery by a bamboo-structured bioceramic with controlled release of hydroxyapatite nanoparticles. Bioactive Materials, 2022, 17: 379.
DOI PMID |
| [17] |
ZHANG H, ZHANG H, XIONG Y, et al. Development of hierarchical porous bioceramic scaffolds with controlled micro/nano surface topography for accelerating bone regeneration. Material Science and Engineering C: Materials Biological Application, 2021, 130: 112437.
DOI URL |
| [18] |
LIU X, HOU W, HE L, et al. AMOT130/YAP pathway in topography-induced BMSC osteoblastic differentiation. Colloids Surface B: Biointerfaces, 2019, 182: 110332.
DOI URL |
| [19] | ZHANG Y, WANG X, LI Y, et al. Cell osteogenic bioactivity mediated precisely by varying scaled micro-pits on ordered micro/nano hierarchical structures of titanium. Regeneration Biomaterials, 2022, 9: rbac046. |
| [20] |
CIAPETTI G, DI POMPO G, AVNET S, et al. Osteoclast differentiation from human blood precursors on biomimetic calcium-phosphate substrates. Acta Biomaterialia, 2017, 50: 102.
DOI PMID |
| [21] |
BAI L, CHEN P, ZHAO Y, et al. A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration. Biomaterials, 2021, 278: 121162.
DOI URL |
| [22] |
GAO Q, HOU Y, LI Z, et al. mTORC2 regulates hierarchical micro/nano topography-induced osteogenic differentiation via promoting cell adhesion and cytoskeletal polymerization. Journal of Cellular and Molecular Medicine, 2021, 25(14): 6695.
DOI URL |
| [23] |
YU X, XU R, ZHANG Z, et al. Different cell and tissue behavior of micro-/nano-tubes and micro-/nano-nets topographies on selective laser melting titanium to enhance osseointegration. International Journal of Nanomedicine, 2021, 16: 3329.
DOI PMID |
| [24] |
LI P, LI Y, KWOK T, et al. A bi-layered membrane with micro-nano bioactive glass for guided bone regeneration. Colloids and Surfaces B: Biointerfaces, 2021, 205: 111886.
DOI URL |
| [25] | WEI W, LI G, WANG J, et al. Involvement of Rac1 in the micro/nano-topography sensing and osteogenic differentiation in bone marrow mesenchymal stem cells. Materials & Design, 2018, 157: 402. |
| [26] |
LONG E G, BULUK M, GALLAGHER M B, et al. Human mesenchymal stem cell morphology, migration, and differentiation on micro and nano-textured titanium. Bioactive Materials, 2019, 4: 249.
DOI PMID |
| [27] |
LI Y, LIU C. Nanomaterial-based bone regeneration. Nanoscale, 2017, 9(15): 4862.
DOI PMID |
| [28] |
LI X, LIU M, CHEN F, et al. Design of hydroxyapatite bioceramics with micro-/nano-topographies to regulate the osteogenic activities of bone morphogenetic protein-2 and bone marrow stromal cells. Nanoscale, 2020, 12(13): 7284.
DOI PMID |
| [29] |
LI X, ZHOU Q, WU Y, et al. Enhanced bone regenerative properties of calcium phosphate ceramic granules in rabbit posterolateral spinal fusion through a reduction of grain size. Bioactive Materials, 2022, 11: 90.
DOI PMID |
| [30] |
ZHAO C, WANG X, GAO L, et al. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomaterialia, 2018, 73: 509.
DOI PMID |
| [31] |
XIA L, LIN K, JIANG X, et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials, 2014, 35(30): 8514.
DOI PMID |
| [32] | ZHU Y, ZHANG K, ZHAO R, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials, 2017: 133. |
| [33] |
ZHAO R, CHEN S, YUAN B, et al. Healing of osteoporotic bone defects by micro-/nano-structured calcium phosphate bioceramics. Nanoscale, 2019, 11(6): 2721.
DOI PMID |
| [34] |
CHENG B, LIU Y, ZHAO Y, et al. The role of anthrax toxin protein receptor 1 as a new mechanosensor molecule and its mechanotransduction in BMSCs under hydrostatic pressure. Scientific Reports, 2019, 9(1): 12642.
DOI PMID |
| [35] |
LIU Z, CAI M, ZHANG X, et al. Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation. Advanced Materials, 2021, 33(51): 2106317.
DOI URL |
| [36] |
DIAZ-RODRIGUEZ P, SáNCHEZ M, LANDIN M. Drug-loaded biomimetic ceramics for tissue engineering. Pharmaceutics, 2018, 10(4): 272.
DOI URL |
| [37] |
HE Y, LI Z, DING X, et al. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin β1/FAKpY397/MAPK pathway. Bioactive Materials, 2022, 8: 109.
DOI URL |
| [38] |
CHEN F, WANG M, WANG J, et al. Effects of hydroxyapatite surface nano/micro-structure on osteoclast formation and activity. Journal of Material Chemistry B, 2019, 7(47): 7574.
DOI URL |
| [39] |
ZHANG X, HUANG P, JIANG G, et al. A novel magnesium ion- incorporating dual-crosslinked hydrogel to improve bone scaffold- mediated osteogenesis and angiogenesis. Material Science and Engineering C: Materials in Biological Application, 2021, 121: 111868.
DOI URL |
| [40] |
CHEN W, XIAO W, LIU X, et al. Pharmacological manipulation of macrophage autophagy effectively rejuvenates the regenerative potential of biodegrading vascular graft in aging body. Bioactive Materials, 2022, 11: 283.
DOI PMID |
| [41] |
YANG C, ZHAO C, WANG X, et al. Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation. Nanoscale, 2019, 11(38): 17699.
DOI URL |
| [42] |
LIU W, ZHANG G, WU J, et al. Insights into the angiogenic effects of nanomaterials: mechanisms involved and potential applications. Journal of Nanobiotechnology, 2020, 18(1): 9.
DOI PMID |
| [43] |
YANG Y, LIN Y, ZHANG Z, et al. Micro/nano-net guides M2-pattern macrophage cytoskeleton distribution via Src-ROCK signalling for enhanced angiogenesis. Biomaterials Science, 2021, 9(9): 3334.
DOI URL |
| [44] | TIAN T, XIE W, GAO W, et al. Micro-nano bioactive glass particles incorporated porous scaffold for promoting osteogenesis and angiogenesis in vitro. Frontier in Chemistry, 2019, 7: 186. |
| [45] |
LIU X, MIAO Y, LIANG H, et al. 3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo. Bioactive Materials, 2022, 12: 120.
DOI URL |
| [46] |
ZHANG T, JIANG M, YIN X, et al. The role of autophagy in the process of osseointegration around titanium implants with micro- nano topography promoted by osteoimmunity. Scientific Reports, 2021, 11(1): 18418.
DOI |
| [47] |
CHEN Z, BACHHUKA A, WEI F, et al. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale, 2017, 9(46): 18129.
DOI PMID |
| [48] | FERRAZ N, HONG J, SANTIN M, et al. Nanoporosity of alumina surfaces induces different patterns of activation in adhering monocytes/macrophages. International Journal of Biomaterials, 2010, 2010: 402715. |
| [49] |
PUJARI S, HOESS A, SHEN J, et al. Effects of nanoporous alumina on inflammatory cell response. Journal of Biomedical Materials Research. Part A, 2014, 102(11): 3773.
DOI PMID |
| [50] |
CHEN Z, NI S, HAN S, et al. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale, 2016, 9(2): 706.
DOI URL |
| [51] |
SUN X, SU W, MA X, et al. Comparison of the osteogenic capability of rat bone mesenchymal stem cells on collagen, collagen/hydroxyapatite, hydroxyapatite and biphasic calcium phosphate. Regenerative Biomaterials, 2018, 5(2): 93.
DOI PMID |
| [52] |
LIAO C, LI Y, TJONG S C. Polyetheretherketone and its composites for bone replacement and regeneration. Polymers (Basel), 2020, 12(12): 2858.
DOI URL |
| [53] |
KAZIMIERCZAK P, PRZEKORA A. Bioengineered living bone grafts-a concise review on bioreactors and production techniques in vitro. International Journal of Molecular Science, 2022, 23(3): 1765.
DOI URL |
| [54] |
CUI N, DAI C Y, MAO X, et al. Poloxamer-based scaffolds for tissue engineering applications: a review. Gels, 2022, 8(6): 360.
DOI URL |
| [55] | WEI S, MA J X, XU L, et al. Biodegradable materials for bone defect repair. Militaryl Medical Research, 2020, 7(1): 54. |
| [56] |
RAMíREZ C, BELMONTE M, MIRANZO P, et al. Applications of ceramic/graphene composites and hybrids. Materials (Basel), 2021, 14(8): 2071.
DOI URL |
| [57] |
LI M, XIONG P, YAN F, et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioactive Materials, 2018, 3(1): 1.
DOI PMID |
| [58] |
BRåNEMARK R, EMANUELSSON L, PALMQUIST A, et al. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine, 2011, 7(2): 220.
DOI PMID |
| [59] |
HUANG L, CAI B, HUANG Y, et al. Comparative study on 3D printed Ti6Al4V scaffolds with surface modifications using hydrothermal treatment and microarc oxidation to enhance osteogenic activity. ACS Omega, 2021, 6(2): 1465.
DOI PMID |
| [60] |
ZHAO L, MEI S, CHU P K, et al. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials, 2010, 31(19): 5072.
DOI PMID |
| [61] |
UENO T, TSUKIMURA N, YAMADA M, et al. Enhanced bone- integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy. Biomaterials, 2011, 32(30): 7297.
DOI URL |
| [62] |
COCKERILL I, SU Y, LEE J H, et al. Micro-/nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization. Nano Letter, 2020, 20(6): 4594.
DOI URL |
| [63] |
WANG X, ZHOU Y, XIA L, et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids and Surfaces B: Biointerfaces, 2015, 126: 358.
DOI URL |
| [64] |
ZHOU C, XU A T, WANG D D, et al. The effects of Sr-incorporated micro/nano rough titanium surface on rBMSC migration and osteogenic differentiation for rapid osteointegration. Biomaterials Science, 2018, 6(7): 1946.
DOI PMID |
| [65] |
WANG F, SHI L, HE W X, et al. Bioinspired micro/nano fabrication on dental implant-bone interface. Applied Surface Science, 2013, 265: 480.
DOI URL |
| [66] |
DING D, XIE Y, LI K, et al. Micro/nano structural tantalum coating for enhanced osteogenic differentiation of human bone marrow stem cells. Materials (Basel), 2018, 11(4): 546.
DOI URL |
| [67] |
RODRIGUEZ-FLOREZ N, GARCIA-TUNON E, MUKADAM Q, et al. An investigation of the mineral in ductile and brittle cortical mouse bone. Journal of Bone and Mineral Research, 2015, 30(5): 786.
DOI URL |
| [68] |
PEREZ-PUYANA V, JIMÉNEZ-ROSADO M, ROMERO A, et al. Polymer-based scaffolds for soft-tissue engineering. Polymers (Basel), 2020, 12(7): 1566.
DOI URL |
| [69] |
SANTOS M I, TUZLAKOGLU K, FUCHS S, et al. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials, 2008, 29(32): 4306.
DOI PMID |
| [70] |
KWAK S, HAIDER A, GUPTA K C, et al. Micro/nano multilayered scaffolds of plga and collagen by alternately electrospinning for bone tissue engineering. Nanoscale Research Letters, 2016, 11(1): 323.
DOI PMID |
| [71] | GONG M, HUANG C, HUANG Y, et al. Core-sheath micro/ nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomedicine: Nanotechnology, Biology and Medicine, 2019, 17: 124. |
| [72] |
CUI L, ZHANG N, CUI W, et al. A novel nano/micro-fibrous scaffold by melt-spinning method for bone tissue engineering. Journal of Bionic Engineering, 2015, 12(1): 117.
DOI URL |
| [73] |
XU A, LIU X, GAO X, et al. Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone- nanohydroxyapatite biocomposite. Materials Science and Engineering: C, 2015, 48: 592.
DOI URL |
| [74] |
LI J, LI L, ZHOU J, et al. 3D printed dual-functional biomaterial with self-assembly micro-nano surface and enriched nano argentum for antibacterial and bone regeneration. Applied Materials Today, 2019, 17: 206.
DOI URL |
| [75] |
WU H, LIU T, XU Z, et al. Enhanced bacteriostatic activity, osteogenesis and osseointegration of silicon nitride/ polyetherketoneketone composites with femtosecond laser induced micro/ nano structural surface. Applied Materials Today, 2020, 18: 100523.
DOI URL |
| [76] | LIN K, XIA L, GAN J, et al. Tailoring the nanostructured surfaces of hydroxyapatite bioceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation. ACS Applied Materials & Interfaces, 2013, 5(16): 8008. |
| [77] |
ELRAYAH A, ZHI W, FENG S, et al. Preparation of micro/nano- structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials, 2018, 11(9): 1516.
DOI URL |
| [78] | MOORTHI A, SARAVANAN S, SRINIVASAN N, et al. Synthesis, characterization and biological action of nano-bioglass ceramic particles for bone formation. Journal of Biomaterials & Tissue Engineering, 2012, 2(3): 197. |
| [79] |
LIN K, WU C, CHANG J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomaterialia, 2014, 10(10): 4071.
DOI PMID |
| [80] |
FENG C, MA B, XU M, et al. Three-dimensional printing of scaffolds with synergistic effects of micro-nano surfaces and hollow channels for bone regeneration. ACS Biomaterials Science and Engineering, 2021, 7(3): 872.
DOI URL |
| [81] |
TANG W, LIN D, YU Y, et al. Bioinspired trimodal macro/ micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomaterialia, 2016, 32: 309.
DOI URL |
| [82] |
HU Q, JIANG W, LI Y, et al. The effects of morphology on physicochemical properties, bioactivity and biocompatibility of micro-/nano-bioactive glasses. Advanced Powder Technology, 2018, 29(8): 1812.
DOI URL |
| [83] |
ZHANG M, DU H, GUAN Y, et al. Study on the effect of PDA-PLGA scaffold loaded with islet cells for skeletal muscle transplantation in the treatment of diabetes. Front Bioengineering and Biotechnology, 2022, 10: 927348.
DOI URL |
| [84] |
GEHRKE S A, CAVALCANTI DE LIMA J H, RODRIGUEZ F, et al. Microgrooves and microrugosities in titanium implant surfaces: an in vitro and in vivo evaluation. Materials, 2019, 12(8): 1287.
DOI URL |
| [85] |
ZHAO W, LI X, LIU X, et al. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Materials Science and Engineering: C, 2014, 40: 316.
DOI URL |
| [86] |
ZHAO Y, BAI L, ZHANG Y, et al. Type I collagen decorated nanoporous network on titanium implant surface promotes osseointegration through mediating immunomodulation, angiogenesis, and osteogenesis. Biomaterials, 2022, 288: 121684.
DOI URL |
| [87] |
ZHU L, CHEN S, LIU K, et al. 3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin- polydopamine for enhanced osteogenic and anti-inflammatory activities. Chemical Engineering Journal, 2020, 391: 123524.
DOI URL |
| [88] |
PEKARKOVA J, GABLECH I, FIALOVA T, et al. Modifications of parylene by microstructures and selenium nanoparticles: evaluation of bacterial and mesenchymal stem cell viability. Front Bioengineering and Biotechnology, 2021, 9: 782799.
DOI URL |
| [89] |
ZHANG Y, YU W, BA Z, et al. 3D-printed scaffolds of mesoporous bioglass/gliadin/polycaprolactone ternary composite for enhancement of compressive strength, degradability, cell responses and new bone tissue ingrowth. International Journal of Nanomedicine, 2018, 13: 5433.
DOI PMID |
| [90] |
JIAO Y, HUANG L J, DUAN T B, et al. Controllable two-scale network architecture and enhanced mechanical properties of (Ti5Si3+TiBw)/Ti6Al4V composites. Scientific Reports, 2016, 6: 32991.
DOI PMID |
| [91] |
WANG S, YANG Y, WANG R, et al. Mineralization of calcium phosphate controlled by biomimetic self-assembled peptide monolayers via surface electrostatic potentials. Bioactive Materials, 2020, 5(2): 387.
DOI URL |
| [92] | ZHAO R, XIE P, ZHANG K, et al. Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation. Acta Biomaterialia, 2017, 1(59): 338. |
| [93] |
YU C, SCHIMELMAN J, WANG P, et al. Photopolymerizable biomaterials and light-based 3d printing strategies for biomedical applications. Chemical Reviews, 2020, 120(19): 10695.
DOI URL |
| [1] | 陈曦, 袁媛, 谭业强, 刘昌胜. 无机非金属生物材料发展战略研究[J]. 无机材料学报, 2025, 40(5): 449-456. |
| [2] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [3] | 郑嘉乾, 卢霄, 鲁亚杰, 王迎军, 王臻, 卢建熙. 医用生物陶瓷的功能性生物适配机制及应用[J]. 无机材料学报, 2024, 39(1): 1-16. |
| [4] | 黄慧, 陈雨. 材料医学和医学材料[J]. 无机材料学报, 2022, 37(11): 1151-1169. |
| [5] | 王恩典, 常江. 钼掺杂铜硅钙石的制备及其抗菌性能和细胞相容性研究[J]. 无机材料学报, 2021, 36(7): 738-744. |
| [6] | 孙团伟,朱英杰. 一步溶剂热法合成锶掺杂羟基磷灰石超长纳米线[J]. 无机材料学报, 2020, 35(6): 724-728. |
| [7] | 马 铭, 卢伟鹏, 曹霄峰, 毛克亚, 郭燕川. 微米级椭球形掺铕羟基磷灰石的发光和细胞毒性研究[J]. 无机材料学报, 2016, 31(8): 890-896. |
| [8] | 陈 峰, 孙团伟, 漆 超, 吴 进, 崔大祥, 朱英杰. 微波辅助溶剂热法制备磷酸钙微球和多面体[J]. 无机材料学报, 2014, 29(7): 776-780. |
| [9] | 侯维敏, 于 云, 胡学兵, 于 洋, 米 乐, 宋力昕. Al2O3微滤膜的超疏水改性研究[J]. 无机材料学报, 2013, 28(8): 864-868. |
| [10] | 吕晓迎, 黄 炎, 俞亚东, 杨雅敏. 基因/蛋白质组学技术在生物材料生物相容性研究中的应用[J]. 无机材料学报, 2013, 28(1): 21-28. |
| [11] | 施剑林, 陈 雨, 陈航榕. 多功能介孔氧化硅基纳米诊疗剂的研究进展[J]. 无机材料学报, 2013, 28(1): 1-11. |
| [12] | 刘宣勇. 硬组织植入材料表/界面研究进展[J]. 无机材料学报, 2011, 26(1): 1-11. |
| [13] | 狄志勇, 何建平, 周建华, 孙 盾, 王 涛. 有机-无机自组装制备类荷叶结构超疏水涂层及其性能研究[J]. 无机材料学报, 2010, 25(7): 765-769. |
| [14] | 邹俭鹏,阮建明,周忠诚,黄伯云,陈启元. 316L纤维尺寸和含量对HA-ZrO2(CaO)/316L纤维复合生物材料性能的影响[J]. 无机材料学报, 2007, 22(5): 1001-1006. |
| [15] | 李延报,李东旭,翁文剑. 无定形磷酸钙及其在生物医学中的应用[J]. 无机材料学报, 2007, 22(5): 775-782. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||