无机材料学报 ›› 2020, Vol. 35 ›› Issue (3): 352-358.DOI: 10.15541/jim20190397 CSTR: 32189.14.10.15541/jim20190397
所属专题: 2020年环境材料论文精选(一)放射性元素去除; 【虚拟专辑】放射性污染物去除(2020~2021)
收稿日期:2019-08-03
修回日期:2019-09-22
出版日期:2020-03-20
网络出版日期:2019-10-23
作者简介:张志宾(1981-), 男, 副教授. E-mail: zhbzhang@ecut.edu.cn
基金资助:
ZHANG Zhibin,ZHOU Runze,DONG Zhimin,CAO Xiaohong,LIU Yunhai( )
)
Received:2019-08-03
Revised:2019-09-22
Published:2020-03-20
Online:2019-10-23
About author:ZHANG Zhibin(1981-), male, associate professor. E-mail: zhbzhang@ecut.edu.cn
Supported by:摘要:
水热碳吸附材料具有制备工艺简单、合成条件温和、表面易改性等优点。本研究以可溶性淀粉为碳源, 在硝酸铈铵催化作用下, 将丙烯腈开环接枝到淀粉分子上, 通过水热反应和盐酸羟胺还原制备偕胺肟化水热碳(AO-HTC)。结合静态和动态吸附实验, 重点研究了溶液pH、碳酸根和钙离子浓度对AO-HTC吸附铀性能的影响, 通过Thomas和Yoon-Nelson模型探究AO-HTC吸附铀的动态过程。结果表明: 随着pH、碳酸根浓度和钙浓度的增加, AO-HTC吸附铀的容量逐渐降低; 掺杂5wt%AO-HTC土壤柱的穿透点和饱和点体积也随之减小。与纯土壤柱相比, 掺杂5wt%AO-HTC土壤柱的最大吸附容量(qo)和吸附质穿透50%所需的时间(τ)增大了数倍。由此可见, AO-HTC是一种性能优异的可渗透性反应墙(PRB)介质, 有望用于修复铀污染土壤和地下水。
中图分类号:
张志宾, 周润泽, 董志敏, 曹小红, 刘云海. 偕胺肟水热碳对U(VI)-CO3/Ca-U(VI)-CO3的吸附性能研究[J]. 无机材料学报, 2020, 35(3): 352-358.
ZHANG Zhibin, ZHOU Runze, DONG Zhimin, CAO Xiaohong, LIU Yunhai. Adsorption of U(VI)-CO3/Ca-U(VI)-CO3 by Amidoxime-functionalized Hydrothermal Carbon[J]. Journal of Inorganic Materials, 2020, 35(3): 352-358.
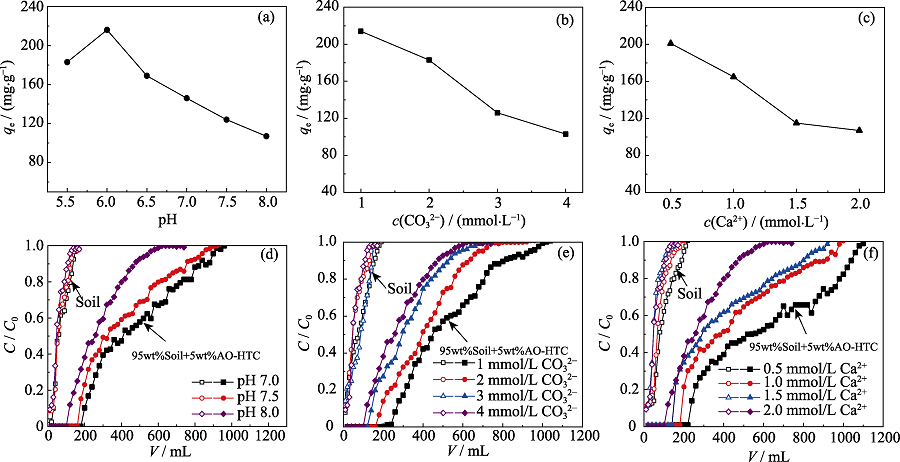
图3 pH(a)、CO32-浓度(b)、Ca2+浓度(c)对AO-HTC吸附铀容量的影响, pH(d)、CO32-浓度(e)、Ca2+浓度(f)对土壤柱和AO-HTC柱穿透曲线的影响
Fig. 3 Effects of pH (a) and CO32 (b) and Ca2+ concentration (c) on uranium adsorbed onto AO-HTC, breakthrough curves of soil column and AO-HTC column at different pH (d), CO32- (e) and Ca2+ concentration (f) (a) 1.0 mmol/L CO32-, 0.5 mmol/L Ca2+; (b) pH=6.0, 0.5 mmol/L Ca2+; (c) pH=6.0, 1.0 mmol/L CO32-; (d) 4.0 mmol/L CO32-, 2.0 mmol/L Ca2+; (e) pH=8.0, 2.0 mmol/L Ca2+; (f) pH=8.0, 4.0 mmol/L CO32-
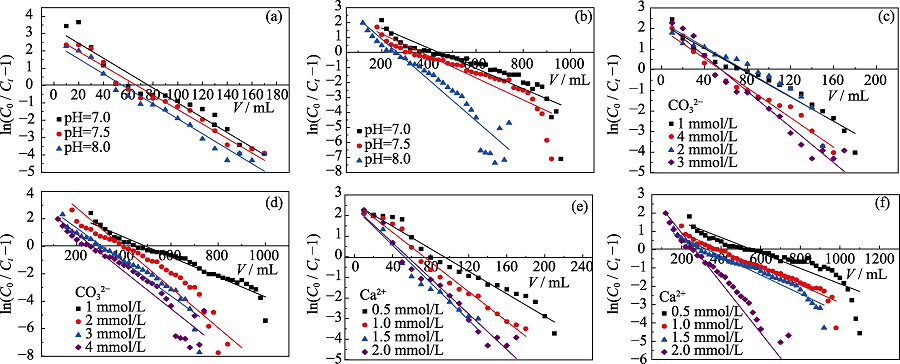
图5 纯土柱(a, c, e)和 AO-HTC柱(b, d, f)在不同条件下的Thomas拟合直线
Fig. 5 Thomas kinetic plots for the adsorption of U(VI) on red soils (a, c, e) and AO-HTC columns (b, d, f) (a, b) Effect of pH; (c, d) Effect of carbonate; (e, f) Effect of calcium (a, b) 4.0 mmol/L CO32- , 2.0 mmol/L Ca2+; (c, d) pH=8.0, 2.0 mmol/L Ca2+; (e, f) pH=8.0, 4.0 mmol/L CO32-
| pH | CO32- /(mmol·L-1) | Ca2+ /(mmol·L-1) | Thomas model | Yoon-Nelson model | |||||
|---|---|---|---|---|---|---|---|---|---|
| KTh /(´10-3, mL·min-1·g-1) | qo /(´10-2, mg∙g-1) | R2 | KYN/min-1 | τ/min | R2 | ||||
| Soil column | 7.0 | 4 | 2 | 2.089 | 1.5 | 0.93 | 0.043 | 76 | 0.93 |
| 7.5 | 2.143 | 1.3 | 0.97 | 0.042 | 67 | 0.97 | |||
| 8.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 7.0 | 4 | 2 | 0.338 | 8.8 | 0.91 | 0.014 | 444 | 0.91 |
| 7.5 | 0.367 | 7.4 | 0.83 | 0.007 | 371 | 0.83 | |||
| 8.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.81 | |||
| Soil column | 8.0 | 1 | 2 | 1.475 | 1.5 | 0.95 | 0.029 | 75 | 0.95 |
| 2 | 1.797 | 1.1 | 0.96 | 0.036 | 55 | 0.96 | |||
| 3 | 1.509 | 1.5 | 0.87 | 0.031 | 76 | 0.87 | |||
| 4 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 1 | 2 | 0.364 | 9.8 | 0.95 | 0.007 | 492 | 0.95 |
| 2 | 0.727 | 7.8 | 0.90 | 0.015 | 392 | 0.90 | |||
| 3 | 0.619 | 6.1 | 0.92 | 0.012 | 307 | 0.92 | |||
| 4 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
| Soil column | 8.0 | 4 | 0.5 | 1.367 | 1.8 | 0.96 | 0.027 | 94 | 0.96 |
| 1.0 | 1.805 | 1.4 | 0.98 | 0.036 | 73 | 0.98 | |||
| 1.5 | 2.275 | 1.0 | 0.95 | 0.046 | 52 | 0.95 | |||
| 2.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 4 | 0.5 | 0.211 | 11.1 | 0.80 | 0.004 | 558 | 0.80 |
| 1.0 | 0.239 | 7.8 | 0.94 | 0.005 | 393 | 0.94 | |||
| 1.5 | 0.257 | 6.6 | 0.94 | 0.005 | 330 | 0.94 | |||
| 2.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
表1 Thomas和Yoon-Nelson模型的拟合参数
Table 1 Parameters of Thomas and Yoon-Nelson models
| pH | CO32- /(mmol·L-1) | Ca2+ /(mmol·L-1) | Thomas model | Yoon-Nelson model | |||||
|---|---|---|---|---|---|---|---|---|---|
| KTh /(´10-3, mL·min-1·g-1) | qo /(´10-2, mg∙g-1) | R2 | KYN/min-1 | τ/min | R2 | ||||
| Soil column | 7.0 | 4 | 2 | 2.089 | 1.5 | 0.93 | 0.043 | 76 | 0.93 |
| 7.5 | 2.143 | 1.3 | 0.97 | 0.042 | 67 | 0.97 | |||
| 8.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 7.0 | 4 | 2 | 0.338 | 8.8 | 0.91 | 0.014 | 444 | 0.91 |
| 7.5 | 0.367 | 7.4 | 0.83 | 0.007 | 371 | 0.83 | |||
| 8.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.81 | |||
| Soil column | 8.0 | 1 | 2 | 1.475 | 1.5 | 0.95 | 0.029 | 75 | 0.95 |
| 2 | 1.797 | 1.1 | 0.96 | 0.036 | 55 | 0.96 | |||
| 3 | 1.509 | 1.5 | 0.87 | 0.031 | 76 | 0.87 | |||
| 4 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 1 | 2 | 0.364 | 9.8 | 0.95 | 0.007 | 492 | 0.95 |
| 2 | 0.727 | 7.8 | 0.90 | 0.015 | 392 | 0.90 | |||
| 3 | 0.619 | 6.1 | 0.92 | 0.012 | 307 | 0.92 | |||
| 4 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
| Soil column | 8.0 | 4 | 0.5 | 1.367 | 1.8 | 0.96 | 0.027 | 94 | 0.96 |
| 1.0 | 1.805 | 1.4 | 0.98 | 0.036 | 73 | 0.98 | |||
| 1.5 | 2.275 | 1.0 | 0.95 | 0.046 | 52 | 0.95 | |||
| 2.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 4 | 0.5 | 0.211 | 11.1 | 0.80 | 0.004 | 558 | 0.80 |
| 1.0 | 0.239 | 7.8 | 0.94 | 0.005 | 393 | 0.94 | |||
| 1.5 | 0.257 | 6.6 | 0.94 | 0.005 | 330 | 0.94 | |||
| 2.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||

图6 纯土柱(a, c, e)和AO-HTC柱(b, d, f)在不同条件下的Yoon-Nelson拟合直线
Fig. 6 Yoon-Nelson kinetic plots for the adsorption of U(VI) on red soils (a, c, e) and AO-HTC columns (b, d, f) (a,b) Effect of pH; (c, d) Effect of calcium; (e, f) Effect of carbonate (a, b) 4.0 mmol/L CO32-, 2.0 mmol/L Ca2+; (c, d) pH=8.0, 2.0 mmol/L Ca2+; (e, f) pH=8.0, 4.0 mmol/L CO32-
| [1] | ZHANG Z B, DONG Z M, WANG X X , et al. Ordered mesoporous polymer-carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chemical Engineering Journal, 2018,341:208-217. |
| [2] | ZENG J Y, ZHANG H, SUI Y , et al. New amidoxime-based material TMP-g-AO for uranium adsorption under seawater conditions. Industrial & Engineering Chemistry Research, 2017,56(17):5021-5032. |
| [3] | LIU Y, CAO X, HUA R , et al. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy, 2010,104(2):150-155. |
| [4] | ZHANG Z, ZHANG H, QIU Y , et al. Study on adsorption of uranium by phosphorylated graphene oxide. Sci. Sin. Chim., 2018,48:1-12. |
| [5] | MAYES R T, GORKA J, DAI S . Impact of pore size on the sorption of uranyl under seawater conditions. Industrial & Engineering Chemistry Research, 2016,55(15):4339-4343. |
| [6] | LI J, WANG X, ZHAO G , et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev., 2018,47(7):2322-2356. |
| [7] | EBBS. Role of uranium speciation in the uptake and translocation of uranium by plants. Journal of Experimental Botany, 1998,49(324):1183-1190. |
| [8] | YAO Z . Review on remediation technologies of soil contaminated by heavy metals. Procedia Environmental Sciences, 2012,16(4):722-731. |
| [9] | LANDSBERGER S, TAMALIS D . Leaching dynamics of uranium in a contaminated soil site. Journal of Radioanalytical & Nuclear Chemistry, 2013,296(1):319-341. |
| [10] | DING D X, LI S M, HU N , et al. Bioreduction of U(VI) in groundwater under anoxic conditions from a decommissioned in situ leaching uranium mine. Bioprocess & Biosystems Engineering, 2015,38(4):661-670. |
| [11] | OBIRI-NYARKO F, GRAJALES-MESA S J, MALINA G . An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere, 2014,111:243-259. |
| [12] | RICHARDSON J P, NICKLOW J W . In situ permeable reactive barriers for groundwater contamination. Soil & Sediment Contamination, 2002,11(2):241-268. |
| [13] | KORNILOVYCH B, WIREMAN M, UBALDINI S , et al. Uranium removal from groundwater by permeable reactive barrier with zero-valent iron and organic carbon mixtures: laboratory and field studies. Metals, 2018,8(6):408-419 |
| [14] | ZHANG W, GUO Y, PAN Z , et al. Remediation of uranium- contaminated groundwater using the permeable reactive barrier thchnique coupled with hydroxyapatite-coated quartz sands. Fresenius Environmental Bulletin, 2018,27(5):2703-2716. |
| [15] | LI Z J, WANG L, YUAN L Y , et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. Journal of Hazardous Materials, 2015,290:26-33. |
| [16] | ZHANG Z, LIU J, CAO X , et al. Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. Journal of Hazardous Materials, 2015,300:633-642. |
| [17] | WILTAFSKY M K, SCHMIDTLEIN B, ROTH F X . Estimates of the optimum dietary ratio of standardized ileal digestible valine to lysine for eight to twenty-five kilograms of body weight pigs. Journal of Animal Science, 2009,87(8):2544-2553. |
| [18] | LIU X, WU J, ZHANG S , et al. Amidoxime-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustainable Chemistry & Engineering, 2019,7(12):10800-10807. |
| [19] | LADSHAW A P, DAS S, LIAO W P , et al. Experiments and modeling of uranium uptake by amidoxime-based adsorbent in the presence of other ions in simulated seawater. Industrial & Engineering Chemistry Research, 2016,55(15):4241-4248. |
| [20] | PANG H B, KUO L J, WAI C M , et al. Elution of uranium and transition metals from amidoxime-based polymer adsorbents for sequestering uranium from seawater. Industrial & Engineering Chemistry Research, 2016,55(15):4313-4320. |
| [21] | HAN B, ZHANG E, CHENG G , et al. Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chemical Engineering Journal, 2018,338:734-744. |
| [22] | ZHANG Z B, DONG Z M, DAI Y , et al. Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution. RSC Advances, 2016,6(104):102462-102471. |
| [23] | YABUSAKI S B, FANG Y, LONG P E , et al. Uranium removal from groundwater via in situ biostimulation: field-scale modeling of transport and biological processes. J. Contam. Hydrol., 2007,93(1-4):216-235. |
| [24] | OMIDI M H, AZAD F N, GHAEDI M , et al. Synthesis and characterization of Au-NPs supported on carbon nanotubes: application for the ultrasound assisted removal of radioactive UO2 2+ ions following complexation with Arsenazo III: spectrophotometric detection, optimization, isotherm and kinetic study. J. Colloid. Interface Sci., 2017,504:68-77. |
| [25] | ZHANG Z B, LIU Y H, CAO X H , et al. Sorption study of uranium on carbon spheres hydrothermal synthesized with glucose from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 2013,295(3):1775-1782. |
| [26] | HUANG F, XU Y, LIAO S , et al. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials, 2013,6(3):969-980. |
| [27] | ZHAO Y, LI J, ZHAO L , et al. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chemical Engineering Journal, 2014,235:275-283. |
| [28] | ZHANG Z B, LIU J, CAO X H , et al. Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. Journal of Hazardous Materials, 2015,300:633-642. |
| [29] | LIU J, ZHAO C, YUAN G , et al. Adsorption of U(VI) on a chitosan/ polyaniline composite in the presence of Ca/Mg-U(VI)-CO3 complexes. Hydrometallurgy, 2018,175:300-311. |
| [30] | LI X, ZHANG M, LIU Y , et al. Removal of U(VI) in aqueous solution by nanoscale zero-valent iron(nZVI). Water Quality Exposure & Health, 2013,5(1):31-40. |
| [31] | CERRATO J M, BARROWS C J, BLUE L Y , et al. Effect of Ca2+ and Zn2+ on UO2 dissolution rates. Environ. Sci. Technol., 2012,46(5):2731-2737. |
| [32] | KENNEDY D W, FREDRICKSON J K, BROOKS S C , et al. Inhibition of bacterial U(VI) reduction by calcium. Abstracts of the General Meeting of the American Society for Microbiology, 2003,37(9):1850-1858. |
| [33] | EL HAYEK E, TORRES C, RODRIGUEZ-FREIRE L , et al. Effect of calcium on the bioavailability of dissolved uranium(VI) in plant roots under circumneutral pH. Environ. Sci. Technol., 2018,52(22):13089-13098. |
| [34] | CHEN L F, YIN X B, YU Q , et al. Rapid and selective capture of perrhenate anion from simulated groundwater by a mesoporous silica-supported anion exchanger. Microporous Mesoporous Mat., 2019,274:155-162. |
| [35] | WANG X X, CHEN L, WANG L , et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China-Chem., 2019,62(8):933-967. |
| [36] | MAHMOUD M A . Adsorption of U(VI) ions from aqueous solution using silicon dioxide nanopowder. Journal of Saudi Chemical Society, 2018,22(2):229-238. |
| [37] | YAKOUT S M, METWALLY S S, EL-ZAKLA T . Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids. Applied Surface Science, 2013,280(8):745-750. |
| [38] | ZHANG Z B, DONG Z M, WANG X X , et al. Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): batch and fixed-bed column studies. Chemical Engineering Journal, 2019,370:1376-1387. |
| [1] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [2] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [3] | 洪培萍, 梁龙, 吴炼, 马颖康, 庞浩. ZIF-67结构调控及其对盐酸金霉素的吸附性能研究[J]. 无机材料学报, 2025, 40(4): 388-396. |
| [4] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [5] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [6] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [7] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [8] | 郭春霞, 陈伟东, 闫淑芳, 赵学平, 杨傲, 马文. 埃洛石纳米管负载锆氧化物吸附水中砷的研究[J]. 无机材料学报, 2023, 38(5): 529-536. |
| [9] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [10] | 于业帆, 徐玲, 倪忠斌, 施冬健, 陈明清. 普鲁士蓝/生物炭材料的制备及其氨氮吸附机理[J]. 无机材料学报, 2023, 38(2): 205-212. |
| [11] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [12] | 汤亚, 孙盛睿, 樊佳, 杨庆峰, 董满江, 寇佳慧, 刘阳桥. 粉煤灰衍生水合硅酸钙PEI改性及吸附去除Cu(II)与催化降解有机污染物[J]. 无机材料学报, 2023, 38(11): 1281-1291. |
| [13] | 戴洁燕, 冯爱虎, 米乐, 于洋, 崔苑苑, 于云. NaY沸石分子吸附涂层对典型空间污染物的吸附机制研究[J]. 无机材料学报, 2023, 38(10): 1237-1244. |
| [14] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| [15] | 刘城, 赵倩, 牟志伟, 雷洁红, 段涛. 新型铋基SiOCNF复合膜对放射性气态碘的吸附性能[J]. 无机材料学报, 2022, 37(10): 1043-1050. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||