无机材料学报 ›› 2025, Vol. 40 ›› Issue (5): 466-472.DOI: 10.15541/jim20240502 CSTR: 32189.14.jim20240502
所属专题: 【能源环境】化工催化(202506)
郭子玉1,2( ), 朱云洲2(
), 朱云洲2( ), 王力2, 陈健2, 李红1(
), 王力2, 陈健2, 李红1( ), 黄政仁2
), 黄政仁2
收稿日期:2024-12-02
修回日期:2025-01-04
出版日期:2025-05-20
网络出版日期:2025-01-24
通讯作者:
朱云洲, 正高级工程师. E-mail: yunzhouzhu@mail.sic.ac.cn;作者简介:郭子玉(1999-), 女, 硕士研究生. E-mail: guoziyu25863@163.com
基金资助:
GUO Ziyu1,2( ), ZHU Yunzhou2(
), ZHU Yunzhou2( ), WANG Li2, CHEN Jian2, LI Hong1(
), WANG Li2, CHEN Jian2, LI Hong1( ), HUANG Zhengren2
), HUANG Zhengren2
Received:2024-12-02
Revised:2025-01-04
Published:2025-05-20
Online:2025-01-24
Contact:
ZHU Yunzhou, professor. E-mail: yunzhouzhu@mail.sic.ac.cn;About author:GUO Ziyu (1999-), female, Master candidate. E-mail: guoziyu25863@163.com
Supported by:摘要:
多孔碳材料的性能及应用很大程度上取决于其微观孔结构。为便捷、有效地大范围调控多孔碳材料的微观孔结构, 本研究基于聚合诱导相分离(Polymerization-induced Phase Separation, PIPS)法, 以酚醛树脂/乙二醇为原料制备了均相多孔碳, 并系统研究了Zn2+含量以及固化温度对多孔碳微观孔结构的影响。研究发现, 随着固化温度的升高, 多孔碳稳定性降低, 微观孔结构均匀性变差。在90 ℃下固化, 引入Zn2+可以使多孔碳的孔隙率从40.22%增至70.38%, 平均孔径从49.8 nm增至279.4 nm, 中位孔径从107.2 nm增至343.0 nm。随着Zn2+含量的增加, 多孔碳的孔隙率、中位孔径和平均孔径都先增大后减小, 当Zn2+质量分数为1.5%时, 平均孔径达到最大值343.0 nm, 孔隙率达到最大值(70.38±0.37)%。研究指出, 引入Zn2+降低了酚醛树脂中苯酚结构的间位取代反应势垒, 有利于在苯环上构建大环化合物, 显著促进了树脂与乙二醇聚合, 提高了树脂混合物的固化程度及骨架聚合度, 使相分离更加彻底。当Zn2+含量过高时, 树脂混合物的聚合度过高, 导致富醇相难以挥发, 孔径结构变差。研究还发现, 引入Zn2+有助于提高石墨化程度, 使碳骨架更加清晰。本研究为调控多孔碳材料的微观孔结构及制备结构碳化物陶瓷提供了理论基础。
中图分类号:
郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472.
GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol[J]. Journal of Inorganic Materials, 2025, 40(5): 466-472.
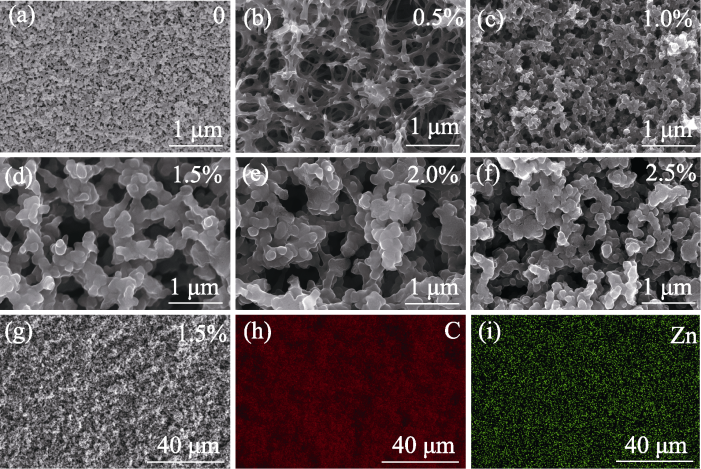
图3 不同Zn2+含量的树脂混合物热解碳化后的微观孔结构(a~g)及EDS元素分布图(h, i)
Fig. 3 Microporous structures (a-g) and EDS element mappings (h, i) of resin mixtures with different Zn2+ contents after carbonation pyrolysis
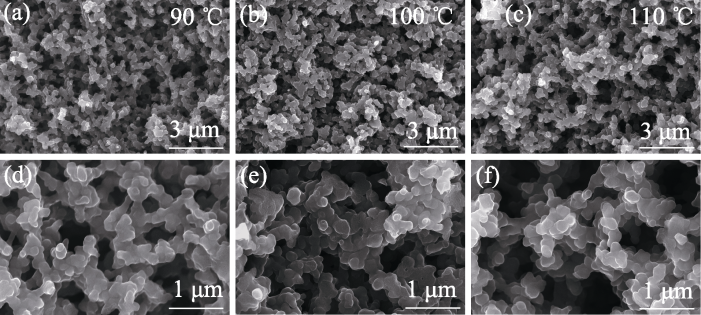
图4 不同温度固化的1.5% Zn2+多孔碳的微观孔结构
Fig. 4 Microporous structures of 1.5% Zn2+ porous carbon cured at different temperatures (a, d) 90 ℃; (b, e) 100 ℃; (c, f) 110 ℃
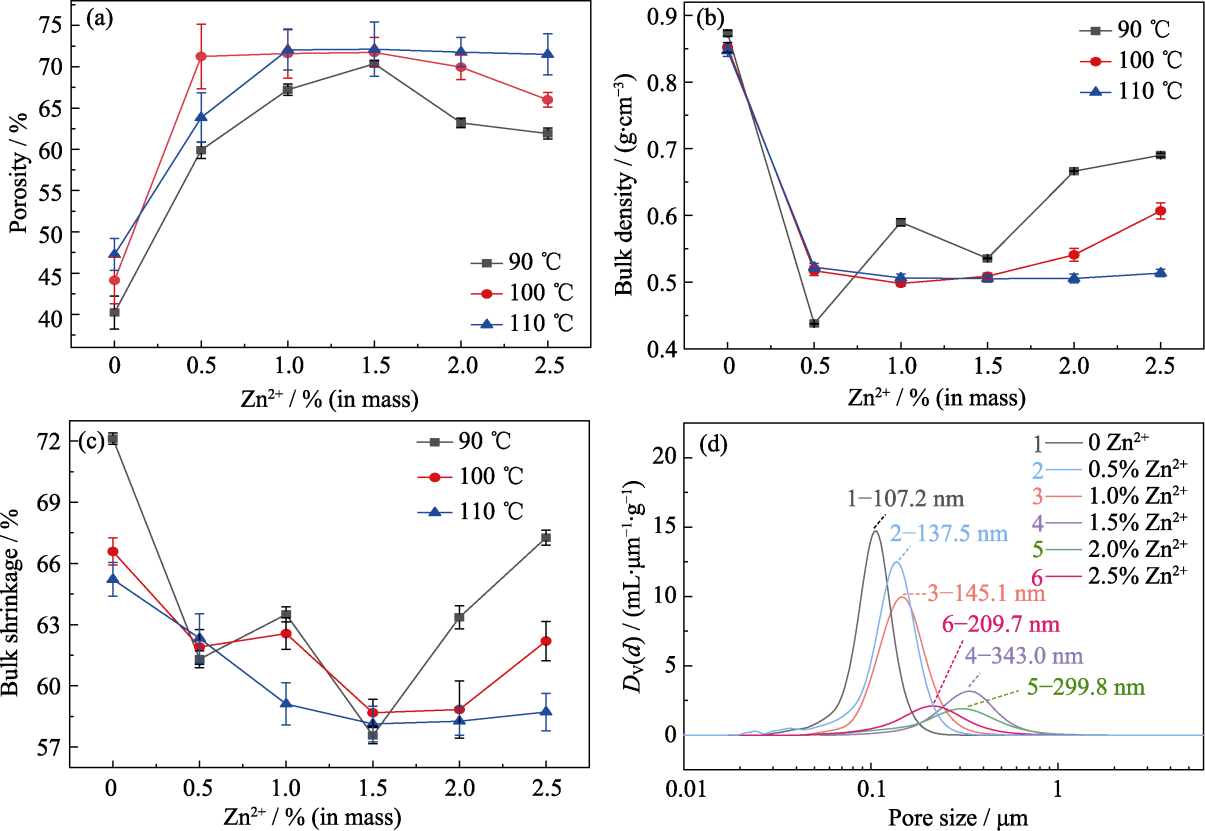
图5 (a~c)多孔碳的(a)孔隙率、(b)体密度、(c)体积收缩率; (d) 90 ℃固化的多孔碳的孔径分布
Fig. 5 (a) Porosity, (b) bulk density and (c) bulk shrinkage of porous carbon; (d) Pore size distribution of porous carbon cured at 90 ℃
| Zn2+/% (in mass) | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 |
|---|---|---|---|---|---|---|
| Mean pore size/nm | 49.8 | 69.1 | 130.0 | 279.4 | 128.0 | 74.3 |
表1 不同Zn2+含量多孔碳的平均孔径
Table 1 Mean pore size of porous carbon with different Zn2+ contents
| Zn2+/% (in mass) | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 |
|---|---|---|---|---|---|---|
| Mean pore size/nm | 49.8 | 69.1 | 130.0 | 279.4 | 128.0 | 74.3 |
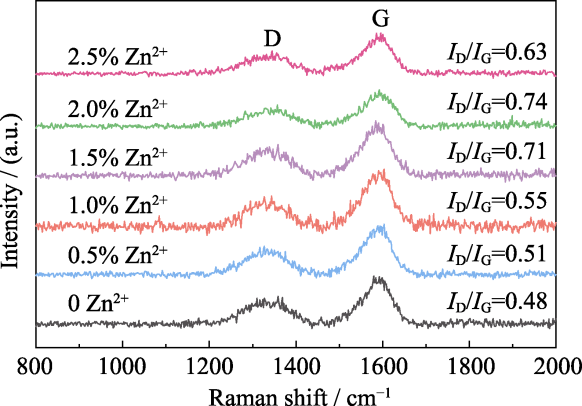
图9 90 ℃固化的不同Zn2+含量树脂混合物热解后多孔碳的拉曼光谱图
Fig. 9 Raman spectra of porous carbon after pyrolysis of resin mixtures with different Zn2+ contents cured at 90 ℃
| [1] | KRAIWATTANAWONG K. A review on the development of a porous carbon-based as modeling materials for electric double layer capacitors. Arabian Journal of Chemistry, 2022, 15(2): 103625. |
| [2] | MEMETOVA A, TYAGI I, KARRI R R, et al. Porous carbon- based material as a sustainable alternative for the storage of natural gas (methane) and biogas (biomethane): a review. Chemical Engineering Journal, 2022, 446: 137373. |
| [3] | ZHANG X, HAN R, LIU Y, et al. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: a review. Chemical Engineering Journal, 2023, 460: 141607. |
| [4] | YANG D, SUN T, TIAN H, et al. Iron-nitrogen-codoped mesoporous carbon: facile synthesis and catalytic performance of oxygen reduction reaction. Journal of Inorganic Materials, 2023, 38(11): 1309. |
| [5] | ZHAO R, TANG S. Research progress of ceramic matrix composites prepared by improved reactive melt infiltration through ceramization of porous carbon matrix. Journal of Inorganic Materials, 2024, 39(6): 623. |
| [6] | WANG Y, CHEN J, IHARA H, et al. Preparation of porous carbon nanomaterials and their application in sample preparation: a review. TrAC Trends in Analytical Chemistry, 2021, 143: 116421. |
| [7] | CHENG X, TANG C, YAN C, et al. Preparation of porous carbon spheres and their application as anode materials for lithium-ion batteries: a review. Materials Today Nano, 2023, 22: 100321. |
| [8] | XIE L, JIN Z, DAI Z, et al. Porous carbons synthesized by templating approach from fluid precursors and their applications in environment and energy storage: a review. Carbon, 2020, 170: 100. |
| [9] | YAN B, ZHENG J, WANG F, et al. Review on porous carbon materials engineered by ZnO templates: design, synthesis and capacitance performance. Materials & Design, 2021, 201: 109518. |
| [10] | XU F, WU D, FU R, et al. Design and preparation of porous carbons from conjugated polymer precursors. Materials Today, 2017, 20(10): 629. |
| [11] | CHEN A, WANG Y, YU Y, et al. Nitrogen-doped hollow carbon spheres for supercapacitors. Journal of Materials Science, 2017, 52(6): 3153. |
| [12] | BHATTACHARJYA D, KIM M S, BAE T S, et al. High performance supercapacitor prepared from hollow mesoporous carbon capsules with hierarchical nanoarchitecture. Journal of Power Sources, 2013, 244: 799. |
| [13] | ZHOU M, LU Y, CHEN H, et al. Excellent durable supercapacitor performance of hierarchical porous carbon spheres with macro hollow cores. Journal of Energy Storage, 2018, 19: 35. |
| [14] | HE Y, ZHUANG X, LEI C, et al. Porous carbon nanosheets: synthetic strategies and electrochemical energy related applications. Nano Today, 2019, 24: 103. |
| [15] | CHEN W, WANG X, FEIZBAKHSHAN M, et al. Preparation of lignin-based porous carbon with hierarchical oxygen-enriched structure for high-performance supercapacitors. Journal of Colloid and Interface Science, 2019, 540: 524. |
| [16] | WANG J, YANG Q, YANG W, et al. Adsorptive catalysis of hierarchical porous heteroatom-doped biomass: from recovered heavy metal to efficient pollutant decontamination. Journal of Materials Chemistry A, 2018, 6(34): 16690. |
| [17] | LEE B M, CHOI B S, LEE J Y, et al. Fabrication of porous carbon beads from polyacrylonitrile as electrode materials for electric double-layer capacitors. Carbon Letters, 2021, 31(1): 67. |
| [18] | XU S, LI J, QIAO G, et al. Pore structure control of mesoporous carbon monoliths derived from mixtures of phenolic resin and ethylene glycol. Carbon, 2009, 47(8):2103. |
| [19] | NAKANISHI K, AMATANI T, YANO S, et al. Multiscale templating of siloxane gels via polymerization-induced phase separation. Chemistry of Materials, 2008, 20(3): 1108. |
| [20] | LUO K. The morphology and dynamics of polymerization-induced phase separation. European Polymer Journal, 2006, 42(7): 1499. |
| [21] | NIKFARJAM N, COMAN P T, FREE C, et al. Advancing ionic conductivity in solid electrolytes: insights from polymerization- induced phase separation and microstructural optimization. Journal of Energy Storage, 2024, 93: 112287. |
| [22] | MANDSBERG N K, ASLAN F, DONG Z, et al. 3D printing of reactive macroporous polymers via thiol-ene chemistry and polymerization-induced phase separation. Chemical Communications, 2024, 60(45): 5872. |
| [23] | SUN H, TAN Y, TANG M, et al. Phenolic resin-based nanoflower porous carbon synthesized by self-assembly of MgO hydrolysis for boosting supercapacitor performance. Applied Surface Science, 2024, 673: 160874. |
| [24] | YUAN Z, ZHANG Y, ZHOU Y, et al. Preparation and characterization of porous carbons obtained from mixtures of furfuryl alcohol and phenol-formaldehyde resin. Materials Chemistry and Physics, 2014, 143(2): 707. |
| [25] | HAN X, YANG W, YIN C, et al. Carbonized derivatives derived from the complex of Fe/Ni bimetallic MOFs and phenolic resin for flexible piezoresistive sensors in motion capture and health monitoring. Applied Surface Science, 2025, 679: 160964. |
| [26] | YU Z L, XIN S, YOU Y, et al. Ion-catalyzed synthesis of microporous hard carbon embedded with expanded nanographite for enhanced lithium/sodium storage. Journal of the American Chemical Society, 2016, 138(45): 14915. |
| [27] | PIZZI A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. Journal of Applied Polymer Science, 1979, 24(5): 1257. |
| [28] | YU Z, LI G, FECHLER N, et al. Polymerization under hypersaline conditions: a robust route to phenolic polymer-derived carbon aerogels. Angewandte Chemie International Edition, 2016, 55(47): 14623. |
| [29] | WU X, ZHU Y, PEI B, et al. Effect of FeCl2 on the pore structure of porous carbon obtained from phenol formaldehyde resin and ethylene glycol. Materials Letters, 2018, 215: 50. |
| [30] | YIN C, HE X, YANG X, et al. Enhanced electrocatalytic removal of bisphenol a by introducing Co/N into precursor formed from phenolic resin waste. Chemosphere, 2024, 358: 142204. |
| [31] | GU J F, CHEN C, CHAEMCHUEN S, et al. Controlled incorporation of Zn into nitrogen-doped porous carbon boosts the alcohol dehydrogenation to carboxylic acids. Materials Today Chemistry, 2024, 40: 102221. |
| [32] | BRUSKO V, KHANNANOV A, RAKHMATULLIN A, et al. Unraveling the infrared spectrum of graphene oxide. Carbon, 2024, 229: 119507. |
| [33] | WEISS S, SEIDL R, KESSLER W, et al. Multivariate curve resolution (MCR) of real-time infrared spectra for analyzing the curing behavior of solid MF thermosetting resin. International Journal of Adhesion and Adhesives, 2021, 110: 102956. |
| [34] | SAHA A, KURREY R, DEB M K, et al. Resin immobilized gold nanocomposites assisted surface enhanced infrared absorption (SEIRA) spectroscopy for improved surface assimilation of methylene blue from aqueous solution. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2021, 262: 120144. |
| [35] | MA Z, MANG C, ZHAO H, et al. Comparison of electromagnetism behavior of different content cobalt-zinc ferrite loaded with graphene. Journal of Inorganic Materials, 2019, 34(4): 407. |
| [36] | HAMDAN M, HALAWY L, HIJAZI A, et al. Highly-stable Ni-Zn catalyst on USY zeolite support for low temperature methane pyrolysis. International Journal of Hydrogen Energy, 2024, 61: 840. |
| [37] | FARID M A A, ZHENG A L T, TSUBOTA T, et al. Catalytic graphitization of biomass-derived ethanosolv lignin using Fe, Co, Ni, and Zn: microstructural and chemical characterization. Journal of Analytical and Applied Pyrolysis, 2023, 173: 106064. |
| [1] | 凌意瀚, 郭胜, 曹志强, 田云峰, 刘方升, 金芳军, 高源. 固体氧化物电池直孔电极结构的制备技术与性能研究进展[J]. 无机材料学报, 2025, 40(12): 1311-1323. |
| [2] | 赵日达, 汤素芳. 多孔碳陶瓷化改进反应熔渗法制备陶瓷基复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 623-633. |
| [3] | 李秋实, 殷广明, 吕伟超, 王怀尧, 李婧琳, 杨红光, 关芳芳. Na+/g-C3N4材料的制备及光催化降解亚甲基蓝机理[J]. 无机材料学报, 2024, 39(10): 1143-1150. |
| [4] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [5] | 马润东, 郭雄, 施凯旋, 安胜利, 王瑞芬, 郭瑞华. MoS2/g-C3N4 S型异质结的构建及光催化性能研究[J]. 无机材料学报, 2023, 38(10): 1176-1182. |
| [6] | 吴西士, 朱云洲, 黄庆, 黄政仁. 树脂基多孔碳孔结构对Cf/SiC复合材料连接性能的影响[J]. 无机材料学报, 2022, 37(12): 1275-1280. |
| [7] | 程晓昆, 张越, 吕海军, 刘歆颖, 侯森林, 陈爱兵. 多孔碳纳米材料构建抗肿瘤药物靶向传递系统的研究进展[J]. 无机材料学报, 2021, 36(1): 9-24. |
| [8] | 许伟佳, 邱大平, 刘诗强, 李敏, 杨儒. 用于高性能超级电容器电极的栓皮栎基多孔活性炭的制备[J]. 无机材料学报, 2019, 34(6): 625-632. |
| [9] | 罗世强, 郑春满, 孙巍巍, 谢威, 柯剑煌, 刘双科, 洪晓斌, 李宇杰, 许静. ZIF-67衍生Co-NC多孔碳材料的可控制备及其在锂-硫二次电池中的应用研究[J]. 无机材料学报, 2019, 34(5): 502-508. |
| [10] | 聂兰舰, 顾真安, 王玉芬, 向在奎, 张辰阳, 饶传东. SiO2疏松体真空烧结致密化与透明化机理研究[J]. 无机材料学报, 2019, 34(10): 1060-1066. |
| [11] | 郝燕霞, 钱猛, 徐吉健, 毕辉, 黄富强. 棉花基多孔碳材料的合成、微结构及超电性能研究[J]. 无机材料学报, 2018, 33(1): 93-99. |
| [12] | 李 君, 曹亚丽, 王鲁香, 贾殿赠. 煤基球形多孔碳用于锂离子电池负极材料的性能研[J]. 无机材料学报, 2017, 32(9): 909-915. |
| [13] | 孙 涛, 杨 琪, 喻佳瑜, 马金鑫. ZnO-C三维网络结构涂层的制备及其储锂性能研究[J]. 无机材料学报, 2017, 32(5): 483-488. |
| [14] | 马鹏飞, 李日红, 张 龙. 溶胶-凝胶法制备高比表面积铝磷钙生物活性玻璃[J]. 无机材料学报, 2017, 32(1): 107-112. |
| [15] | 徐国忠, 金文武, 曾燮榕, 邹继兆, 熊信柏, 黄 麟, 赵振宁. 煤基炭泡沫孔结构调控[J]. 无机材料学报, 2016, 31(9): 961-968. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||