
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (1): 61-69.DOI: 10.15541/jim20240176
Special Issue: 【能源环境】污染物催化去除(202506); 【信息功能】MAX、MXene及其他二维材料(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
LIU Huilai1,2( ), LI Zhihao1,2, KONG Defeng1,2, CHEN Xing1,2(
), LI Zhihao1,2, KONG Defeng1,2, CHEN Xing1,2( )
)
Received:2024-04-10
Revised:2024-06-20
Published:2025-01-20
Online:2024-06-24
Contact:
CHEN Xing, professor. E-mail: xingchen@hfut.edu.cnAbout author:LIU Huilai (1996-), male, PhD candidate. E-mail: 1141749101@qq.com
Supported by:CLC Number:
LIU Huilai, LI Zhihao, KONG Defeng, CHEN Xing. Preparation of FePc/MXene Composite Cathode and Electro-Fenton Degradation of Sulfadimethoxine[J]. Journal of Inorganic Materials, 2025, 40(1): 61-69.
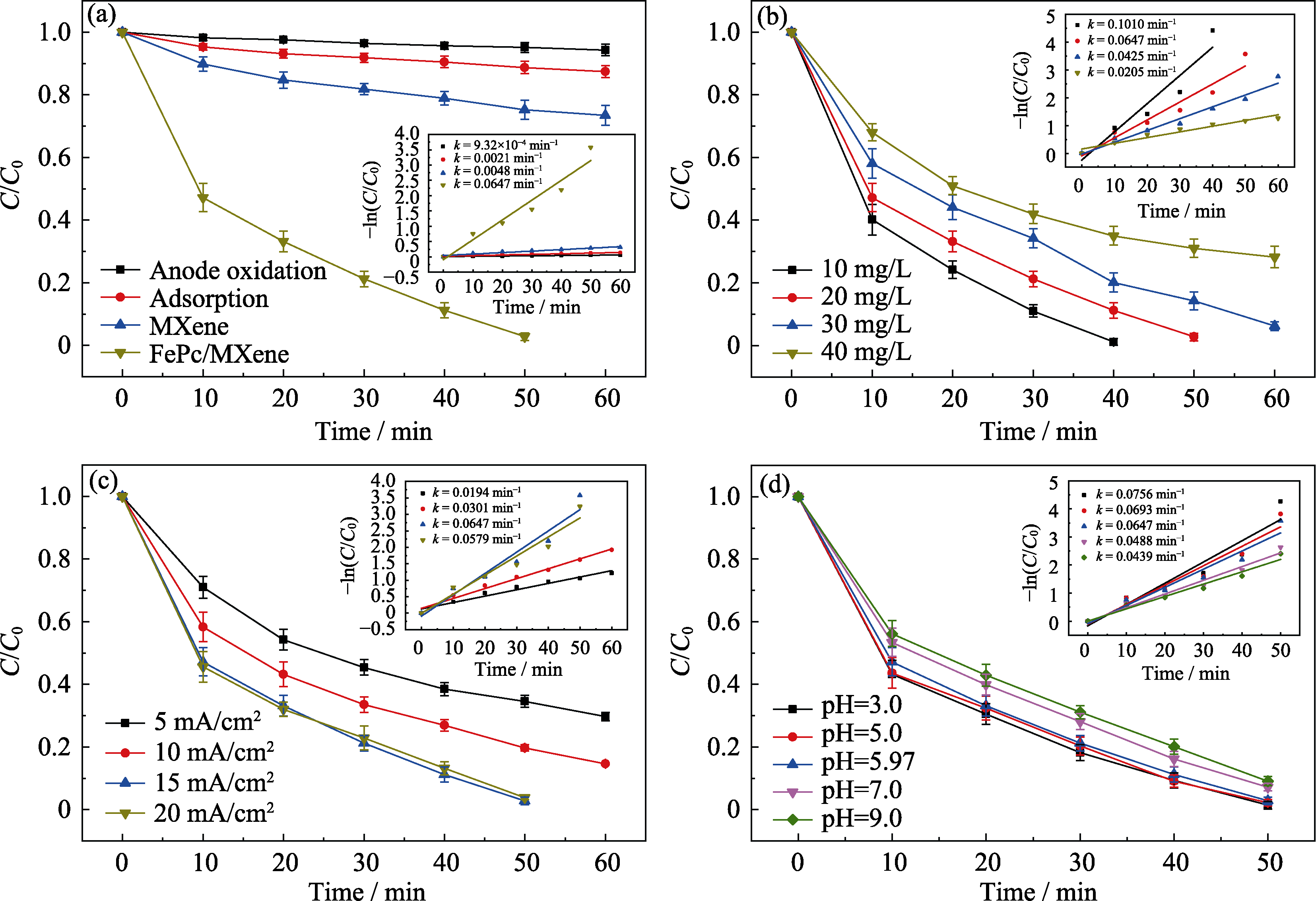
Fig. 6 SDM degradation rates and kinetics curves in the electro-Fenton system under different experimental conditions (a) Different cathode materials; (b) Initial SDM concentration; (c) Current density; (d) Initial pH
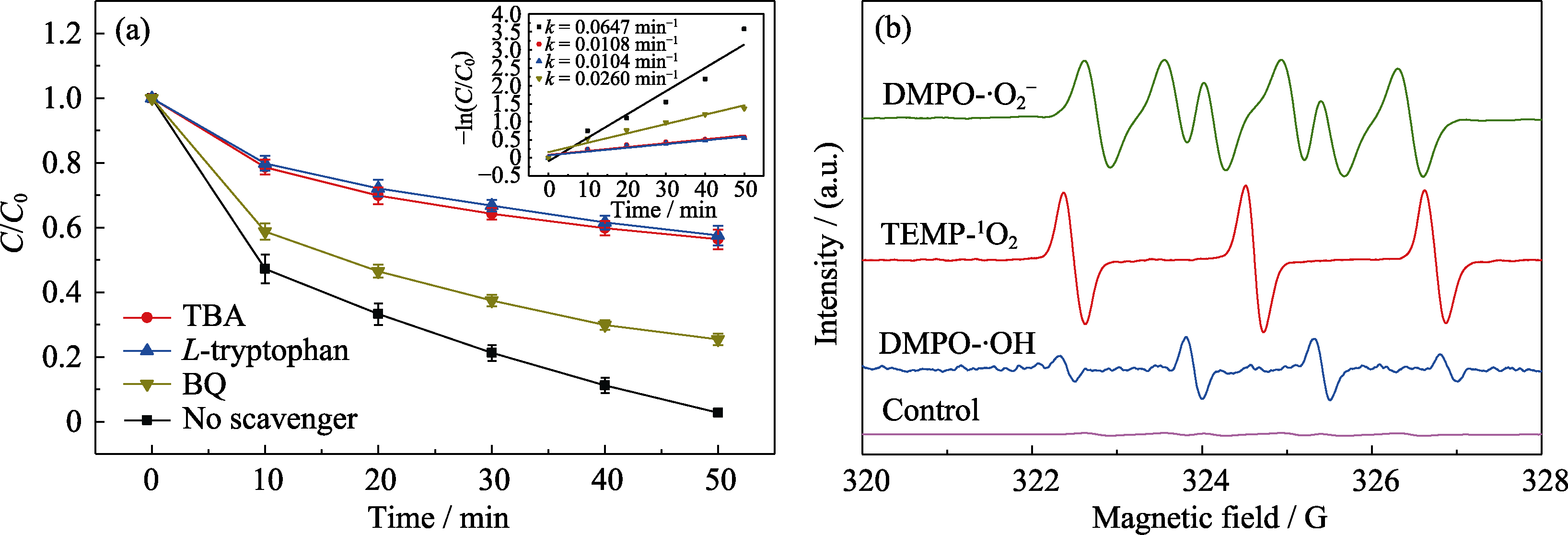
Fig. 7 (a) Effects of BQ, TBA and L-tryptophan radical scavengers on SDM degradation, and (b) ESR spectra of ·OH, ·O2- and 1O2 generated by catalyst in the electro-Fenton system

Fig. 8 Molecular geometry optimization of SDM (a) Optimized geometric structure; (b) HOMO and (c) LUMO distributions; (d) Molecular electrostatic potential; (e) Calculated Fukui index; (f) Degradation pathways of SDM
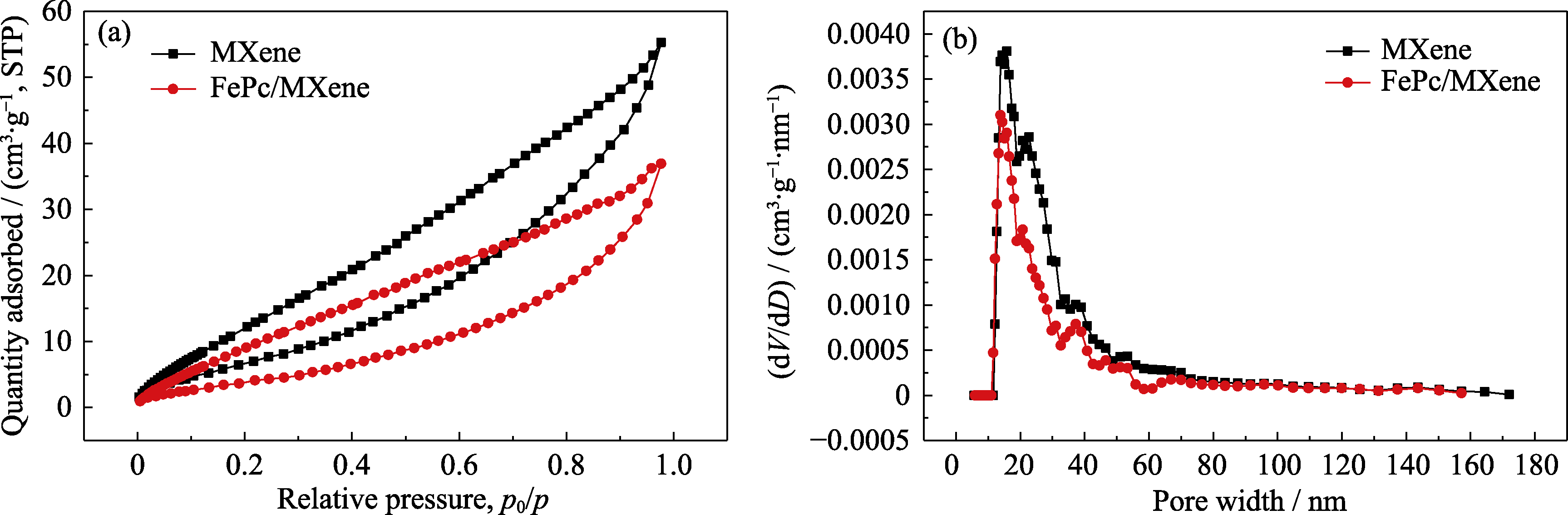
Fig. S1 N2 adsorption-desorption isotherms (a) and corresponding pore size distribution curves (b) of MXene and FePc/MXene Colorful figure is available on website
| Sample | Shell | CN | R/Å | σ2/(×10-3, Å2) | ΔE0/eV | R factor/% |
|---|---|---|---|---|---|---|
| FePc | Fe-N | 4.0 | 1.93 | 7.9 | 3.2 | 1.8 |
| FePc/MXene | Fe-N | 4.1 | 1.96 | 1.1 | 5.1 | 1.5 |
Table S1 EXAFS fitting parameters of Fe K-edge in FePc and FePc/MXene
| Sample | Shell | CN | R/Å | σ2/(×10-3, Å2) | ΔE0/eV | R factor/% |
|---|---|---|---|---|---|---|
| FePc | Fe-N | 4.0 | 1.93 | 7.9 | 3.2 | 1.8 |
| FePc/MXene | Fe-N | 4.1 | 1.96 | 1.1 | 5.1 | 1.5 |
| [1] | KEBEDE T G, DUBE S, NINDI M M. Application of mesoporous nanofibers as sorbent for removal of veterinary drugs from water systems. Science of the Total Environment, 2020, 738: 140282. |
| [2] | ZHOU M H, TAN Q Q, WANG Q, et al. Degradation of organics in reverse osmosis concentrate by electro-Fenton process. Journal of Hazardous Materials, 2012, 215/216: 287. |
| [3] |
BEDIOUI F, GRIVEAU S, NYOKONG T, et al. Tuning the redox properties of metalloporphyrin- and metallophthalocyanine-based molecular electrodes for the highest electrocatalytic activity in the oxidation of thiols. Physical Chemistry Chemical Physics, 2007, 9(26): 3383.
DOI PMID |
| [4] | FENG Y C, WANG X, WANG D. Metal porphyrins and metal phthalocyanines as designable molecular model electrocatalysts. Materials Chemistry Frontiers, 2024, 8(1): 228. |
| [5] | LI M, LI Z L, YU X L, et al. FeN4-doped carbon nanotubes derived from metal organic frameworks for effective degradation of organic dyes by peroxymonosulfate: impacts of FeN4 spin states. Chemical Engineering Journal, 2022, 431: 133339. |
| [6] | TANIGUCHI T, TATEISHI H, MIYAMOTO S, et al. A self-assembly route to an iron phthalocyanine/reduced graphene oxide hybrid electrocatalyst affording an ultrafast oxygen reduction reaction. Particle & Particle Systems Characterization, 2013, 30(12): 1063. |
| [7] | XIA M T, CHEN B J, GU F, et al. Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance. ACS Nano, 2020, 14(4): 5111. |
| [8] | WANG S Y, FENG A H, LI X Y, et al. Pb(II) adsorption process of Fe3O4supported Ti3C2Tx. Journal of Inorganic Materials, 2023, 38(5): 521. |
| [9] | WEN Y Y, RUFFORD T E, CHEN X Z, et al. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy, 2017, 38: 368. |
| [10] |
LI N, KONG Z Z, CHEN X Z, et al. Research progress of novel two-dimensional materials in photocatalysis and electrocatalysis. Journal of Inorganic Materials, 2020, 35(7): 735.
DOI |
| [11] | CHEN C, WANG T H, ZHAO X D, et al. Customizing hydrophilic terminations for V2CTx MXene toward superior hybrid-ion storage in aqueous zinc batteries. Advanced Functional Materials, 2024, 34(9): 2308508. |
| [12] | FEI L, LEI L, WANG D G. Progress of two-dimensional MXene in new-type thin-film solar cells. Journal of Inorganic Materials, 2024, 39(2): 215. |
| [13] | XUE Q, ZHANG H, ZHU M, et al. Photoluminescent Ti3C2 MXene quantum dots for multicolor cellular imaging. Advanced Materials, 2017, 29(15): 1604847. |
| [14] | ZOROMBA M S, ALHARBI F, AL-HOSSAINY A F, et al. Preparation of hybrid conducting polymers blend nanocomposite for energy conversion using experimental data and TD-DFT/DMOl3 computations. Journal of Materials Research and Technology, 2023, 23: 2852. |
| [15] | ZHANG H L, LI M, ZHU C X, et al. Preparation of magnetic α-Fe2O3/ZnFe2O4@Ti3C2 MXene with excellent photocatalytic performance. Ceramics International, 2020, 46(1): 81. |
| [16] | ZHANG H L, LI M, CAO J L, et al. 2D a-Fe2O3 doped Ti3C2 MXene composite with enhanced visible light photocatalytic activity for degradation of Rhodamine B. Ceramics International, 2018, 44(16): 19958. |
| [17] | ZHAO Y X, LIU J X, ZHANG T, et al. Synthesis of novel hollow carbon nanotubes@Co-Fe alloy/iron phthalocyanine electrocatalyst by self-assembly method for OER and ORR study. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 684: 133093. |
| [18] | DING Z B, XU X T, LI J B, et al. Nanoarchitectonics from 2D to 3D: MXenes-derived nitrogen-doped 3D nanofibrous architecture for extraordinarily-fast capacitive deionization. Chemical Engineering Journal, 2022, 430: 133161. |
| [19] | LI T F, YAO L L, LIU Q L, et al. Fluorine-free synthesis of high-purity Ti3C2Tx (T=OH, O) via alkali treatment. Angewandte Chemie International Edition, 2018, 57(21): 6115. |
| [20] | LI L F, WEN Y D, HAN G K, et al. Tailoring the stability of Fe-N-C via pyridinic nitrogen for acid oxygen reduction reaction. Chemical Engineering Journal, 2022, 437: 135320. |
| [21] | MIČUŠÍK M, ŠLOUF M, STEPURA A, et al. Aging of 2D MXene nanoparticles in air: an XPS and TEM study. Applied Surface Science, 2023, 610: 155351. |
| [22] | ZHANG Q, GUO D, YANG Y L, et al. Enhanced electrocatalytic performance of 2D/2D MXene/reduced graphene oxide (RGO) heterostructures for paracetamol (APAP) degradation: the critical contributions of charge transfer and Ti-OH edges for active species generation. Journal of Environmental Chemical Engineering, 2023, 11(3): 110193. |
| [23] | LI Z L, ZHUANG Z C, LV F, et al. The marriage of the FeN4 moiety and MXene boosts oxygen reduction catalysis: Fe3d electron delocalization matters. Advanced Materials, 2018, 30(43): 1803220. |
| [24] | GAO M S, SUN Y, ZHAO K, et al. FePc/MXene as an efficient catalyst for the selective electroreduction of CO2 into CO in a flow cell. Journal of Environmental Chemical Engineering, 2024, 12(1): 111802. |
| [25] | XIAO M L, XING Z H, JIN Z, et al. Preferentially engineering FeN4 edge sites onto graphitic nanosheets for highly active and durable oxygen electrocatalysis in rechargeable Zn-air batteries. Advanced Materials Interfaces, 2020, 32(49): e2004900. |
| [26] | CHEN G B, AN Y, LIU S W, et al. Highly accessible and dense surface single metal FeN4 active sites for promoting the oxygen reduction reaction. Energy & Environmental Science, 2022, 15(6): 2619. |
| [27] | LIU Y, WEERASOORIYA R, CHEN X. The metal-organic framework supported gold nanoparticles as a highly sensitive platform for electrochemical detection of methyl mercury species in the aqueous environment. Journal of Hazardous Materials, 2022, 431: 128608. |
| [28] |
FRANZ J A, WILLIAMS R J, FLORA J R V, et al. Electrolytic oxygen generation for subsurface delivery: effects of precipitation at the cathode and an assessment of side reactions. Water Research, 2002, 36(9): 2243.
PMID |
| [29] |
LU T, CHEN Q X. Van der Waals potential: an important complement to molecular electrostatic potential in studying intermolecular interactions. Journal of Molecular Modeling, 2020, 26(11): 315.
DOI PMID |
| [1] | WANG Lei, LI Jianjun, NING Jun, HU Tianyu, WANG Hongyang, ZHANG Zhanqun, WU Linxin. Enhanced Degradation of Methyl Orange with CoFe2O4@Zeolite Catalyst as Peroxymonosulfate Activator: Performance and Mechanism [J]. Journal of Inorganic Materials, 2023, 38(4): 469-476. |
| [2] | CHEN Yi-Fan, TANG Xiao-Ning, ZHANG Bin, LUO Yong, LI Yang. TiO2@SiO2 Composites: Preparation and Photocatalytic Antimicrobial Performance [J]. Journal of Inorganic Materials, 2019, 34(12): 1325-1333. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||