
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (1): 53-60.DOI: 10.15541/jim20240140
Special Issue: 【能源环境】氢能材料(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
LIAN Minli( ), SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin(
), SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin( )
)
Received:2024-03-22
Revised:2024-07-19
Published:2025-01-20
Online:2024-07-26
Contact:
LI Dalin, professor. E-mail: dalinli@fzu.edu.cnAbout author:LIAN Minli (1999-), female, Master candidate. E-mail: 975216049@qq.com
Supported by:CLC Number:
LIAN Minli, SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin. Supported Ni Catalysts from Ni-Mg-Al Hydrotalcite-like Compounds:Preparation and Catalytic Performance for Ammonia Decomposition[J]. Journal of Inorganic Materials, 2025, 40(1): 53-60.

Fig. 1 (A) XRD patterns and (B) SEM images of the as-synthesized NixMg75-xAl25 HTlc precursors (a) Ni5Mg70Al25-HTlc; (b) Ni10Mg65Al25-HTlc; (c) Ni15Mg60Al25-HTlc; (d) Ni20Mg55Al25-HTlc; (e) Ni25Mg50Al25-HTlc
| Sample | Metal content/(%, in atom) | Lattice parameter/nm | |||
|---|---|---|---|---|---|
| Ni | Mg | Al | a | c | |
| Ni5Mg70Al25-HTlc | 5.5 | 68.1 | 26.4 | 0.306 | 2.344 |
| Ni10Mg65Al25-HTlc | 10.4 | 63.1 | 26.5 | 0.305 | 2.344 |
| Ni15Mg60Al25-HTlc | 15.9 | 58.4 | 25.7 | 0.306 | 2.344 |
| Ni20Mg55Al25-HTlc | 20.5 | 53.5 | 26.0 | 0.305 | 2.344 |
| Ni25Mg50Al25-HTlc | 25.6 | 48.9 | 25.5 | 0.305 | 2.347 |
Table 1 Metal contents and lattice parameters of the NixMg75-xAl25 HTlc precursors
| Sample | Metal content/(%, in atom) | Lattice parameter/nm | |||
|---|---|---|---|---|---|
| Ni | Mg | Al | a | c | |
| Ni5Mg70Al25-HTlc | 5.5 | 68.1 | 26.4 | 0.306 | 2.344 |
| Ni10Mg65Al25-HTlc | 10.4 | 63.1 | 26.5 | 0.305 | 2.344 |
| Ni15Mg60Al25-HTlc | 15.9 | 58.4 | 25.7 | 0.306 | 2.344 |
| Ni20Mg55Al25-HTlc | 20.5 | 53.5 | 26.0 | 0.305 | 2.344 |
| Ni25Mg50Al25-HTlc | 25.6 | 48.9 | 25.5 | 0.305 | 2.347 |
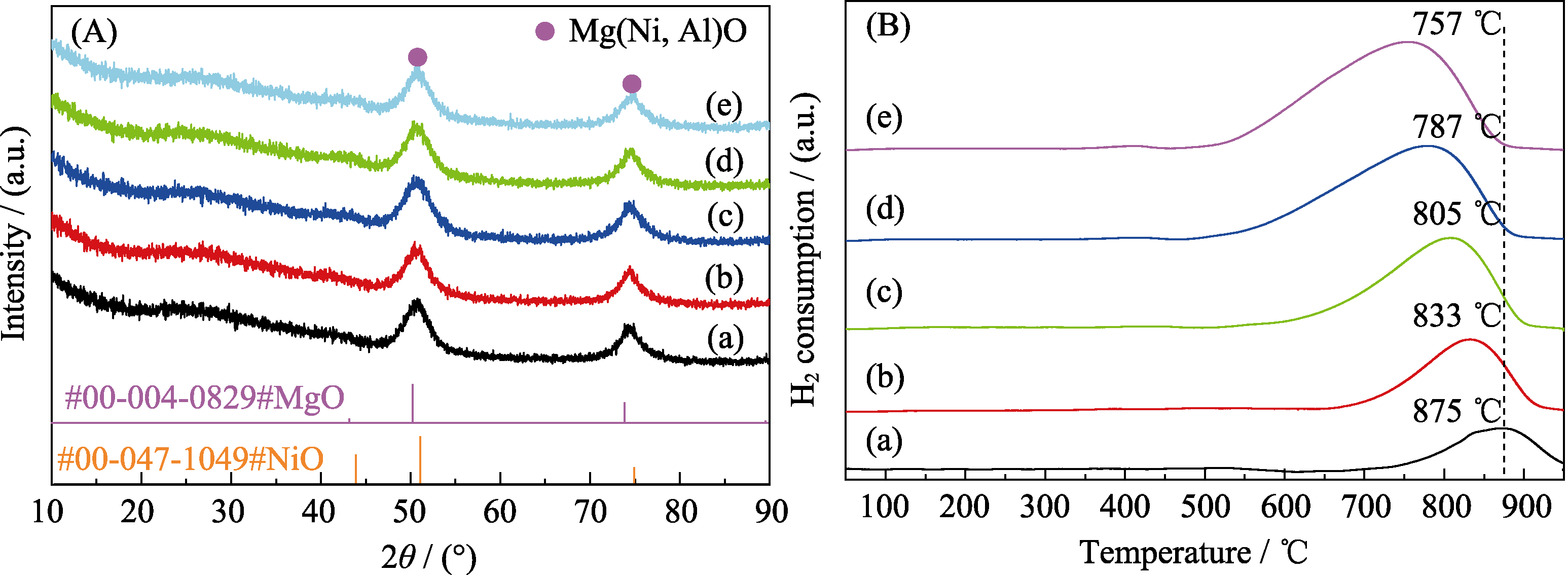
Fig. 2 (A) XRD patterns and (B) H2-TPR profiles of calcined NixMg75-xAl25 (a) Ni5Mg70Al25; (b) Ni10Mg65Al25; (c) Ni15Mg60Al25; (d) Ni20Mg55Al25; (e) Ni25Mg50Al25
| Sample | Surface area/ (m2·gcat-1) | Pore volume/ (cm3·gcat-1) | Pore diameter/ nm | Ni crystal size/ nm | |
|---|---|---|---|---|---|
| Ni5Mg70Al25 | 197.3 | 0.45 | 7.5 | 7.7 | |
| Ni10Mg65Al25 | 173.0 | 0.31 | 5.6 | 7.6 | |
| Ni15Mg60Al25 | 217.4 | 0.36 | 5.6 | 5.9 | |
| Ni20Mg55Al25 | 218.6 | 0.38 | 5.5 | 5.9 | |
| Ni25Mg50Al25 | 204.4 | 0.43 | 7.0 | 6.7 | |
Table 2 Textural properties of the calcined samples and crystallite size of Ni metal in the reduced catalysts
| Sample | Surface area/ (m2·gcat-1) | Pore volume/ (cm3·gcat-1) | Pore diameter/ nm | Ni crystal size/ nm | |
|---|---|---|---|---|---|
| Ni5Mg70Al25 | 197.3 | 0.45 | 7.5 | 7.7 | |
| Ni10Mg65Al25 | 173.0 | 0.31 | 5.6 | 7.6 | |
| Ni15Mg60Al25 | 217.4 | 0.36 | 5.6 | 5.9 | |
| Ni20Mg55Al25 | 218.6 | 0.38 | 5.5 | 5.9 | |
| Ni25Mg50Al25 | 204.4 | 0.43 | 7.0 | 6.7 | |
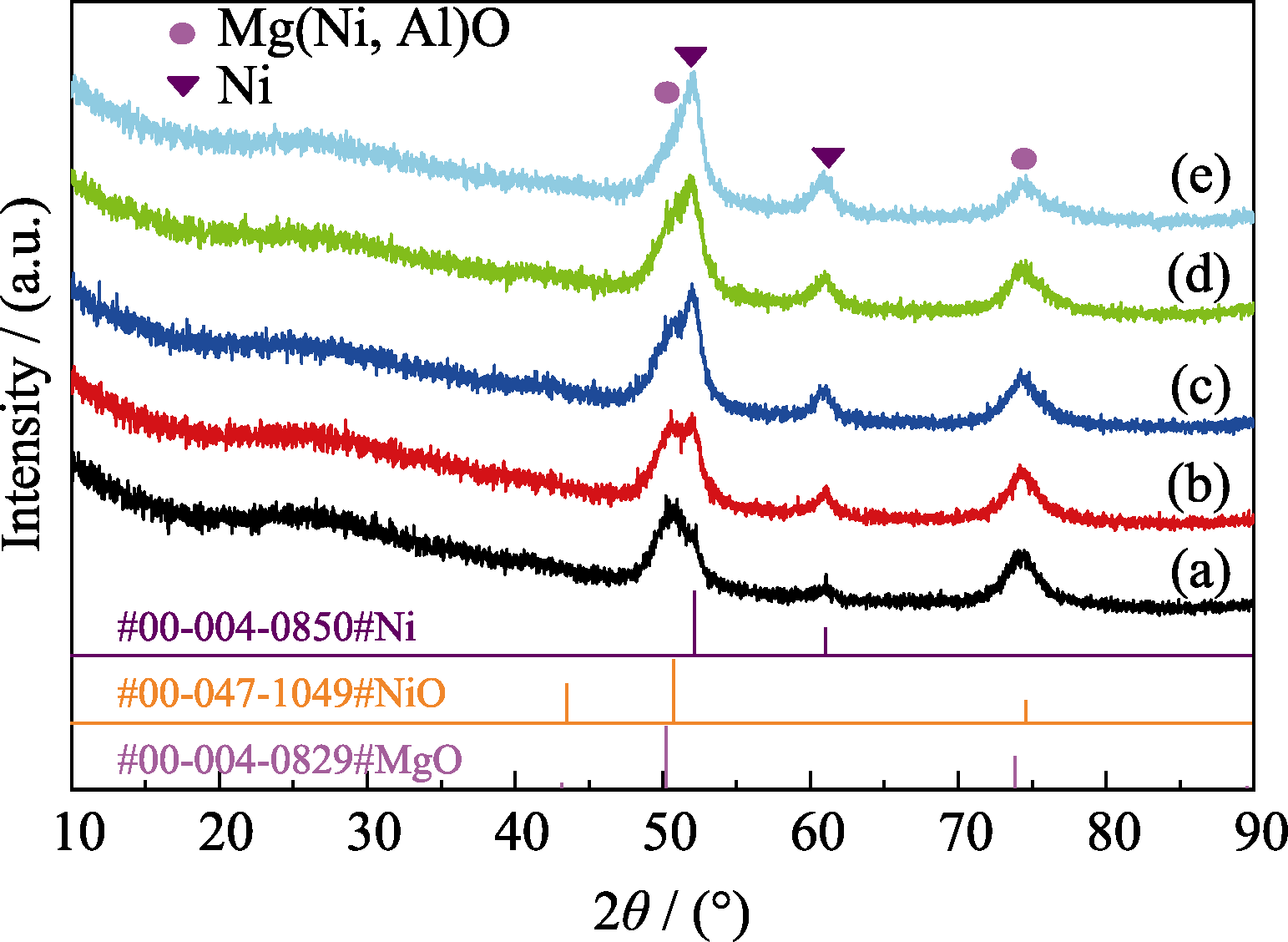
Fig. 4 XRD patterns of NixMg75-xAl25 catalysts reduced at 750 ℃ with NH3 (a) Ni5Mg70Al25; (b) Ni10Mg65Al25; (c) Ni15Mg60Al25; (d) Ni20Mg55Al25; (e) Ni25Mg50Al25

Fig. 5 (a) HAADF-STEM image, (b-f) EDX elemental mappings, (g) TEM image, (h) HRTEM image, and (i) Ni particle size distribution for the Ni20Mg55Al25 catalyst reduced at 750 ℃ with NH3
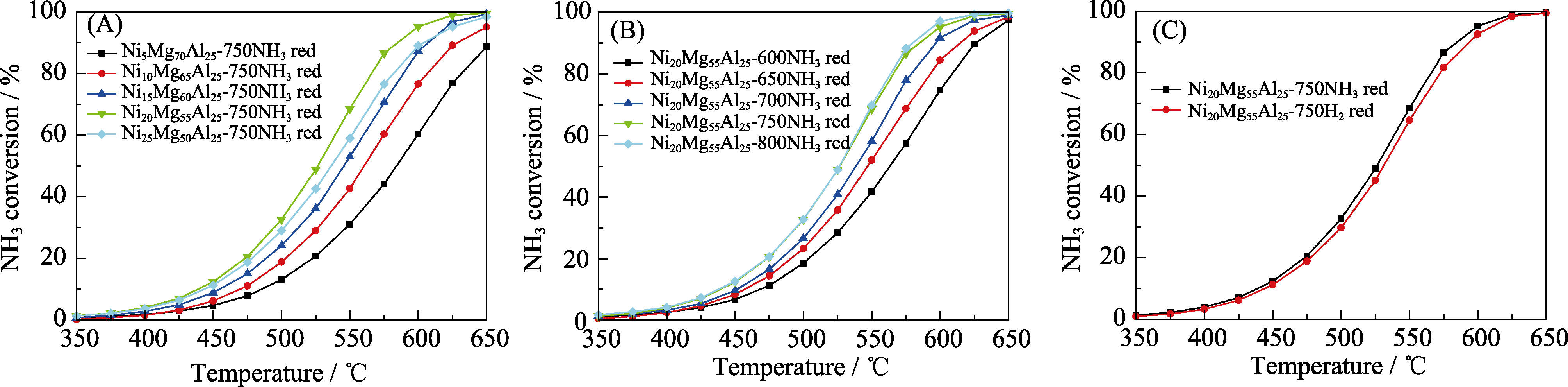
Fig. 6 Catalytic ammonia decomposition over the NixMg75-xAl25 catalysts Influence of (A) Ni contents, (B) reduction temperature, and (C) reduction atmosphere

Fig. 7 (A) Catalytic activities at different space velocities and (B) long-term stability of Ni20Mg55Al25 (pre-reduced with NH3 at 750 ℃), and (C) XRD patterns of the fresh and used Ni20Mg55Al25
| Catalyst | Feed gas | Space velocity/ (mL·gcat-1·h-1) | Temperature/ ℃ | NH3 conversion/ % | NH3 reaction rate/ (mmol· gcat-1·min-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni20Mg55Al25 | Pure NH3 | 30000 | 550 | 68.5 | 15.3 | This work |
| NiCe0.85Zr0.15O | Pure NH3 | 30000 | 550 | 63 | 14.1 | [ |
| Ni/cCeO2(IMP) | Pure NH3 | 30000 | 550 | 51 | 11.4 | [ |
| Ni_MgAl(6:1) | Pure NH3 | 30000 | 550 | 48 | 10.7 | [ |
| Ni/MCM-41(TIE) | Pure NH3 | 30000 | 550 | 47.6 | 10.6 | [ |
| Ni/BaZrO3 | Pure NH3 | 20000 | 550 | 62 | 9.2 | [ |
| Ni1.2Ce0.1Al | Pure NH3 | 45000 | 550 | 26 | 8.7 | [ |
| Ni/La2O3 | Pure NH3 | 6000 | 550 | 78.9 | 3.5 | [ |
Table 3 Catalytic performance of Ni catalysts for ammonia decomposition
| Catalyst | Feed gas | Space velocity/ (mL·gcat-1·h-1) | Temperature/ ℃ | NH3 conversion/ % | NH3 reaction rate/ (mmol· gcat-1·min-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni20Mg55Al25 | Pure NH3 | 30000 | 550 | 68.5 | 15.3 | This work |
| NiCe0.85Zr0.15O | Pure NH3 | 30000 | 550 | 63 | 14.1 | [ |
| Ni/cCeO2(IMP) | Pure NH3 | 30000 | 550 | 51 | 11.4 | [ |
| Ni_MgAl(6:1) | Pure NH3 | 30000 | 550 | 48 | 10.7 | [ |
| Ni/MCM-41(TIE) | Pure NH3 | 30000 | 550 | 47.6 | 10.6 | [ |
| Ni/BaZrO3 | Pure NH3 | 20000 | 550 | 62 | 9.2 | [ |
| Ni1.2Ce0.1Al | Pure NH3 | 45000 | 550 | 26 | 8.7 | [ |
| Ni/La2O3 | Pure NH3 | 6000 | 550 | 78.9 | 3.5 | [ |
| [1] | LAN R, IRVINE J T S, TAO S. Ammonia and related chemicals as potential indirect hydrogen storage materials. International Journal of Hydrogen Energy, 2012, 37(2): 1482. |
| [2] | SCHUTH F, PALKOVITS R, SCHLOGL R, et al. Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy & Environmental Science, 2012, 5(4): 6278. |
| [3] | WAN Z, TAO Y, SHAO J, et al. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Conversion and Management, 2021, 228: 113729. |
| [4] | 王晓光, 韦永德, 张建, 等. 高效镍基氨分解催化体系中载体作用的研究. 石油学报(石油加工), 2006, 22(5): 33. |
| [5] | MAEDA A, HU Z, KUNIMORI K, et al. Effect of high- temperature reduction on ammonia decomposition over niobia- supported and niobia-promoted rhodium catalysts. Catalysis Letters, 1988, 1(5): 155. |
| [6] | JU X, LIU L, YU P, et al. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Applied Catalysis B: Environmental, 2017, 211: 167. |
| [7] | 倪平, 储伟, 罗仕忠, 等. 钡修饰Ir/SiO2催化剂对氨分解促进作用的研究. 合成化学, 2007, 15(4): 407. |
| [8] | PARKER L A, CARTER J H, DUMMER N F, et al. Ammonia decomposition enhancement by Cs-promoted Fe/Al2O3 catalysts. Catalysis Letters, 2020, 150(12): 3369. |
| [9] | MALEKI H, BERTOLA V. Co-Ce-Al-O mesoporous catalysts for hydrogen generation via ammonia decomposition. International Journal of Hydrogen Energy, 2024, 51: 267. |
| [10] | XU J, YAN H, JIN Z, et al. Facile synthesis of stable MO2N nanobelts with high catalytic activity for ammonia decomposition. Chinese Journal of Chemistry, 2019, 37(4): 364. |
| [11] | LI L, WU J, SHAO J, et al. Impacts of SiO2 shell structure of Ni@SiO2 nanocatalysts on their performance for catalytic decomposition of ammonia. Catalysis Letters, 2016, 147(1): 141. |
| [12] | SIMONSEN S B, CHAKRABORTY D, CHORKENDORFF I, et al. Alloyed Ni-Fe nanoparticles as catalysts for NH3 decomposition. Applied Catalysis A: General, 2012, 447/448: 22. |
| [13] | ZANMAN S F, JOLAOSO L A, PODILA S, et al. Ammonia decomposition over citric acid chelated γ-Mo2N and Ni2Mo3N catalysts. International Journal of Hydrogen Energy, 2018, 43(36): 17252. |
| [14] | HE H, JIANG H, YANG F, et al. Bimetallic NixCo10-x/CeO2 as highly active catalysts to enhance mid-temperature ammonia decomposition: kinetics and synergies. International Journal of Hydrogen Energy, 2023, 48(13): 5030. |
| [15] | PODILA S, DRISS H, ZAMAN S F, et al. MgFe and Mg-Co-Fe mixed oxides derived from hydrotalcites: highly efficient catalysts for COx free hydrogen production from NH3. International Journal of Hydrogen Energy, 2020, 45(1): 873. |
| [16] | PANSARE S S, TORRES W, GOODWIN J G. Ammonia decomposition on tungsten carbide. Catalysis Communications, 2007, 8(4): 649. |
| [17] | CHOI J G. Ammonia decomposition over vanadium carbide catalysts. Journal of Catalysis, 1999, 182(1): 104. |
| [18] | OTREMBA T, FRENZEL N, LERCH M, et al. Kinetic studies on ammonia decomposition over zirconium oxynitride. Applied Catalysis A: General, 2011, 392(1): 103. |
| [19] | LE T A, DO Q C, KIM Y, et al. A review on the recent developments of ruthenium and nickel catalysts for COx-free H2 generation by ammonia decomposition. Korean Journal of Chemical Engineering, 2021, 38(6): 1087. |
| [20] | TAKEHIRA K. Recent development of layered double hydroxide- derived catalysts—rehydration, reconstitution, and supporting, aiming at commercial application. Applied Clay Science, 2017, 136: 112. |
| [21] | TAKEHIRA K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg-Al hydrotalcite-like anionic clay. Journal of Catalysis, 2004, 221(1): 43. |
| [22] | SERRANOL A, RODRIGUEZ L, MUNOZ G, et al. Biogas reforming on La-promoted NiMgAl catalysts derived from hydrotalcite-like precursors. Journal of Power Sources, 2011, 196(9): 4404. |
| [23] | BETCHAKU M, NAKAGAWA Y, TAMURA M, et al. Combination of hydrotalcite-like-compound-derived Ni-Fe/Mg/Al and ceria- supported Rh catalysts for fuel reforming in exhaust gas recirculation system of gasoline engine. Fuel Processing Technology, 2022, 225: 107061. |
| [24] | YU X P, CHU W, WANG N, et al. Hydrogen production by ethanol steam reforming on NiCuMgAl catalysts derived from hydrotalcite-like precursors. Catalysis Letters, 2011, 141(8): 1228. |
| [25] | DENG L, LIN H, LIU X, et al. Nickel nanoparticles derived from the direct thermal reduction of Ni-containing Ca-Al layered double hydroxides for hydrogen generation via ammonia decomposition. International Journal of Hydrogen Energy, 2021, 46(77): 38351. |
| [26] | SATO K, ABE N, KAWAGOE T, et al. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. International Journal of Hydrogen Energy, 2017, 42(10): 6610. |
| [27] | SU Q, GU L, YAO Y, et al. Layered double hydroxides derived Nix(MgyAlzOn) catalysts: enhanced ammonia decomposition by hydrogen spillover effect. Applied Catalysis B: Environmental, 2017, 201: 451. |
| [28] | OLSBYE U, AKPORIAYE D, RYTTER E, et al. On the stability of mixed M2+/M3+ oxides. Applied Catalysis A: General, 2002, 224(1): 39. |
| [29] | OKURA K, MIYAZAKI K, MUROYAMA H, et al. Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen. RSC Advances, 2018, 8(56): 32102. |
| [30] | MUROYAMA H, SABURI C, MATSUI T, et al. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Applied Catalysis A: General, 2012, 443/444: 119. |
| [31] | LI X, JI W, ZHAO J, et al.Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. Journal of Catalysis, 2005, 236(2): 181. |
| [32] | ZHENG W, ZHANG J, GE Q, et al. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Applied Catalysis B: Environmental, 2008, 80(1/2): 98. |
| [33] | WU K, CAO C F, ZHOU C, et al. Engineering of Ce3+-O-Ni structures enriched with oxygen vacancies via Zr doping for effective generation of hydrogen from ammonia. Chemical Engineering Science, 2021, 245: 116818. |
| [34] | LIU H, ZHANG Y, LIU S, et al. Ni-CeO2 nanocomposite with enhanced metal-support interaction for effective ammonia decomposition to hydrogen. Chemical Engineering Journal, 2023, 473: 145371. |
| [1] | YU Zelong, TANG Chun, RAO Jiahao, GUO Heng, ZHOU Ying. Preparation and Economic Analysis of High-current-density Electrocatalysts for Alkaline Water Electrolysis [J]. Journal of Inorganic Materials, 2025, 40(12): 1405-1413. |
| [2] | TANG Yang, LIU Limin, ZHOU Xiaoliang, ZHANG Bo, JIANG Xingzhou, JIA Haoyi, LUO Yanlinqing. Proton Ceramic Membrane Reactor: Preparation and Low-temperature Ammonia Decomposition Performance [J]. Journal of Inorganic Materials, 2025, 40(11): 1277-1284. |
| [3] | CHEN Hanxiang, ZHOU Min, MO Zhao, YI Jianjian, LI Huaming, XU Hui. 0D/2D CoN/g-C3N4 Composites: Structure and Photocatalytic Performance for Hydrogen Production [J]. Journal of Inorganic Materials, 2022, 37(9): 1001-1008. |
| [4] | MA Hui, TAO Jianghui, WANG Yanni, HAN Yu, WANG Yabin, DING Xiuping. Gold Nanoparticles Supported on Silica & Titania Hybrid Mesoporous Spheres and Their Catalytic Performance Regulation [J]. Journal of Inorganic Materials, 2022, 37(4): 404-412. |
| [5] | YU Bo, ZHANG Wen-Qiang, LIANG Ming-De, ZHANG Ping, XU Jing-Ming. Effect of PMMA Pore Former on Hydrogen Production Performance of Solid Oxide Electrolysis Cells [J]. Journal of Inorganic Materials, 2011, 26(8): 807-812. |
| [6] | YUAN Wen- Hui, ZHOU Chen-Chen, LI Li. Study on Catalytic Properties of Nano-CeO2-ZrO2 Mixed Oxides Prepared by Modified Sol-Gel Method [J]. Journal of Inorganic Materials, 2010, 25(8): 820-824. |
| [7] |
YAN Jian-Hui,ZHANG Li,ZHU Yi-Rong,TANG You-Gen,YANG Hai-Hua.
Preparation and Photocatalytic Hydrogen Production of NiO(CoO)/N-SrTiO3 Heterojunction Complex Catalyst under Simulated Sunlight Irradiation [J]. Journal of Inorganic Materials, 2009, 24(4): 666-670. |
| [8] | ZHAO Yue-Chang,LIU Ling,CHENG Wen-Ping,WU Hai-Hong,YANG Jian-Guo,HE Ming-Yuan. Hydrothermal Synthesis of MgAlZnFeCe Hydrotalcite-like Precursors and Their Complex Oxides for Application in FCC De-SOx [J]. Journal of Inorganic Materials, 2009, 24(1): 171-174. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||