
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (12): 1281-1288.DOI: 10.15541/jim20220129
• RESEARCH ARTICLE • Previous Articles Next Articles
LUO Yi1( ), XIA Shuhai2, NIU Bo2, ZHANG Yayun2, LONG Donghui2(
), XIA Shuhai2, NIU Bo2, ZHANG Yayun2, LONG Donghui2( )
)
Received:2022-03-08
Revised:2022-05-06
Published:2022-12-20
Online:2022-05-27
Contact:
LONG Donghui, professor. E-mail: longdh@ecust.edu.cnAbout author:LUO Yi (1993-), male, PhD candidate. E-mail: sosolyi@163.com
CLC Number:
LUO Yi, XIA Shuhai, NIU Bo, ZHANG Yayun, LONG Donghui. Preparation and High Temperature Inorganic Transformation of Flexible Silicone Aerogels[J]. Journal of Inorganic Materials, 2022, 37(12): 1281-1288.
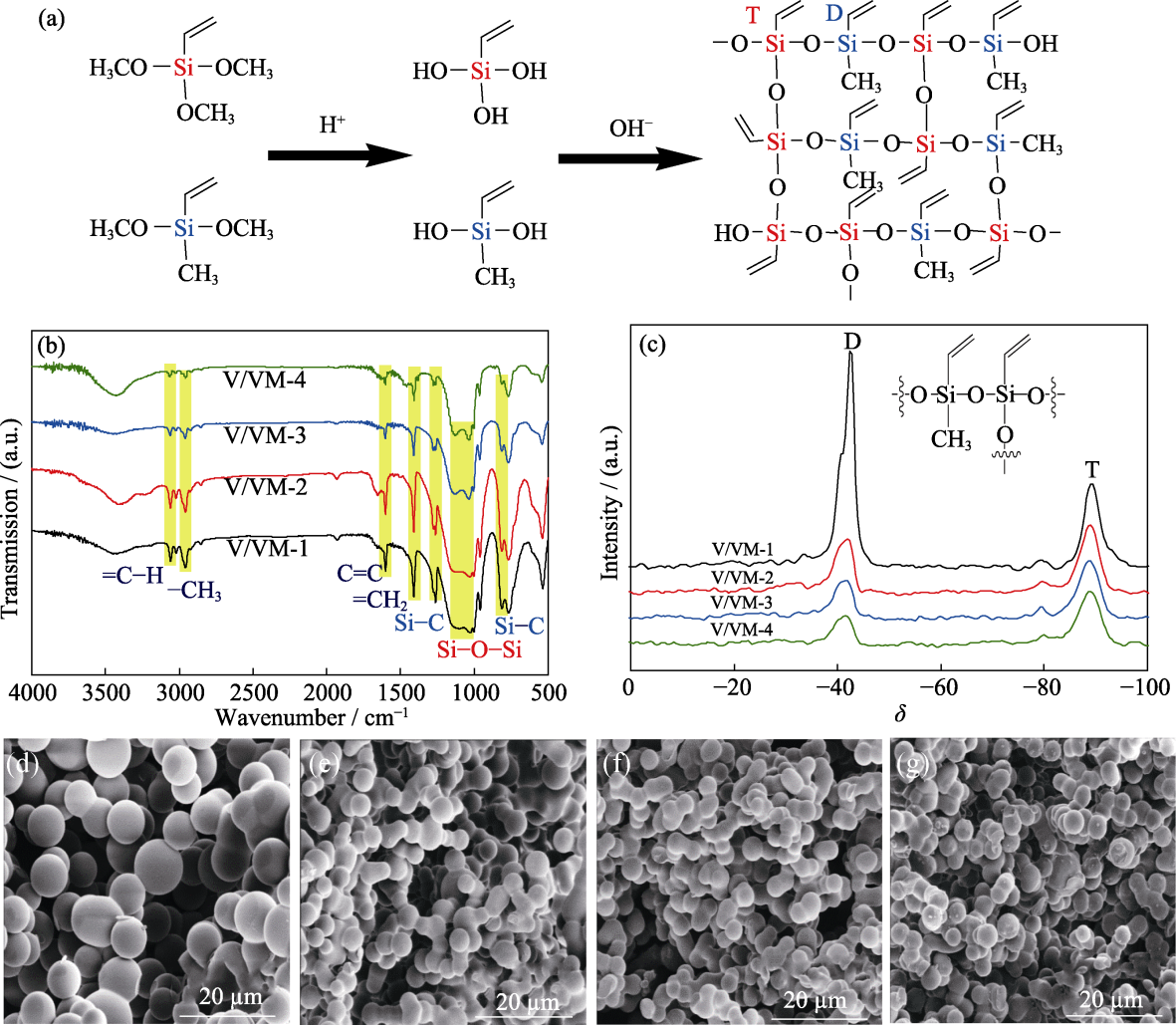
Fig. 1 Schematic diagram of Sol-Gel process (a), IR spectra (b), NMR spectra (c) and SEM images (d-g) of aerogel samples Molar ratios of VTMS/VMDMS for aerogels (d-g) are 1, 2, 3, 4, respectively
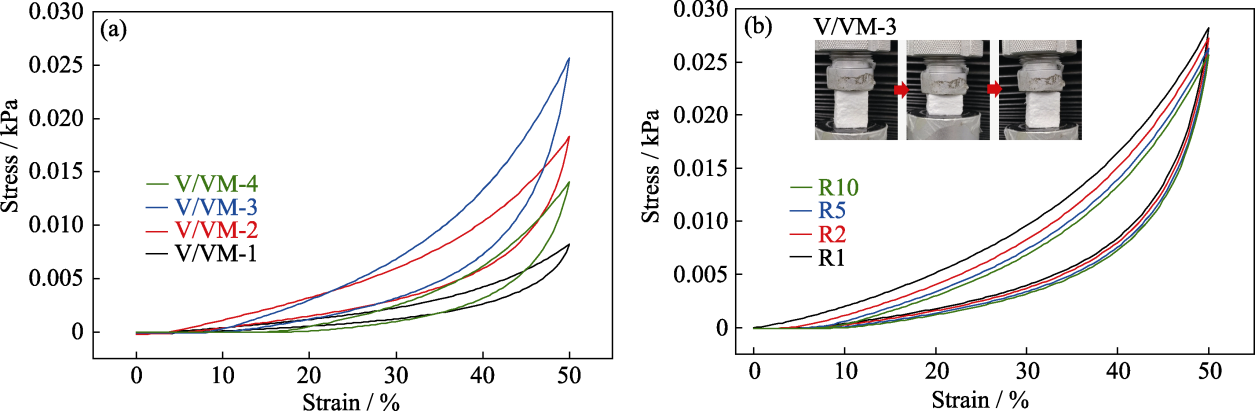
Fig. 2 Cyclic compression stress-strain curves of aerogels (a) Stress-strain curves after 10-cycle compression; (b) Cyclic compression stress-strain curves of sample V/VM-3 Colorful figures are available on website
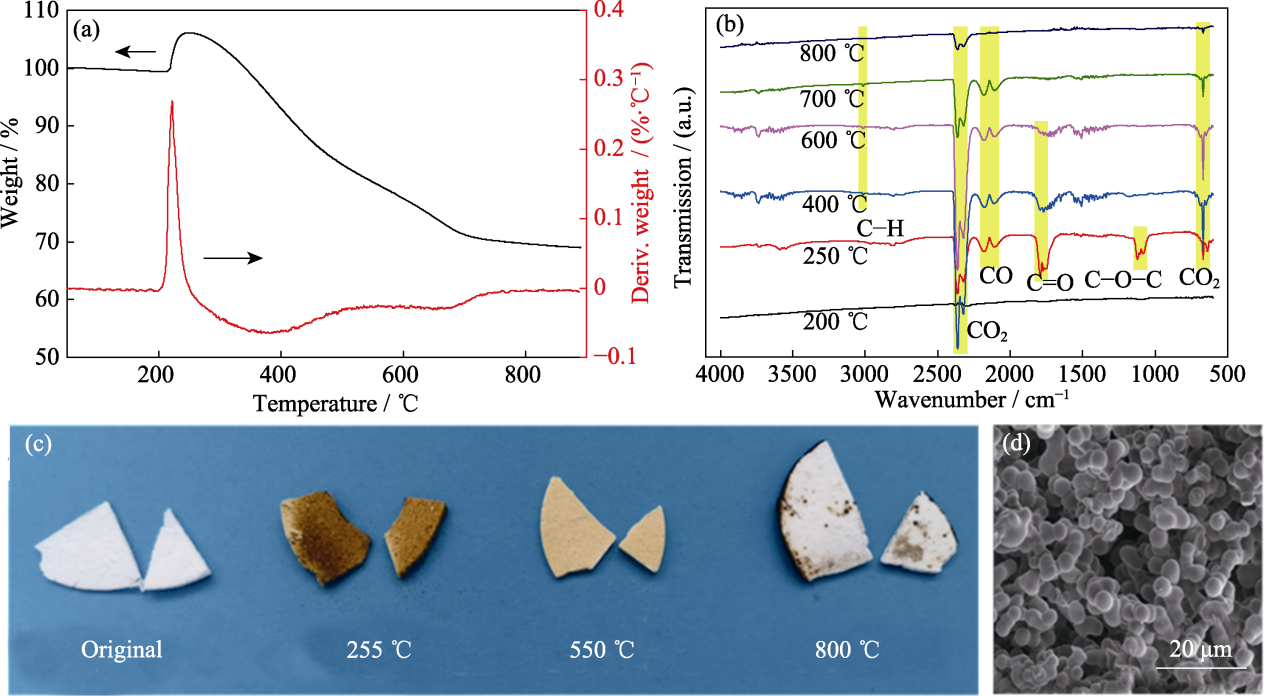
Fig. 3 (a, b) TG-IR spectra of sample V/VM-3 tested in air; (c) Photographs of sample V/VM-3 after heat-treated at different temperatures; (d) SEM image of sample V/VM-3 after heat-treated at 800 ℃
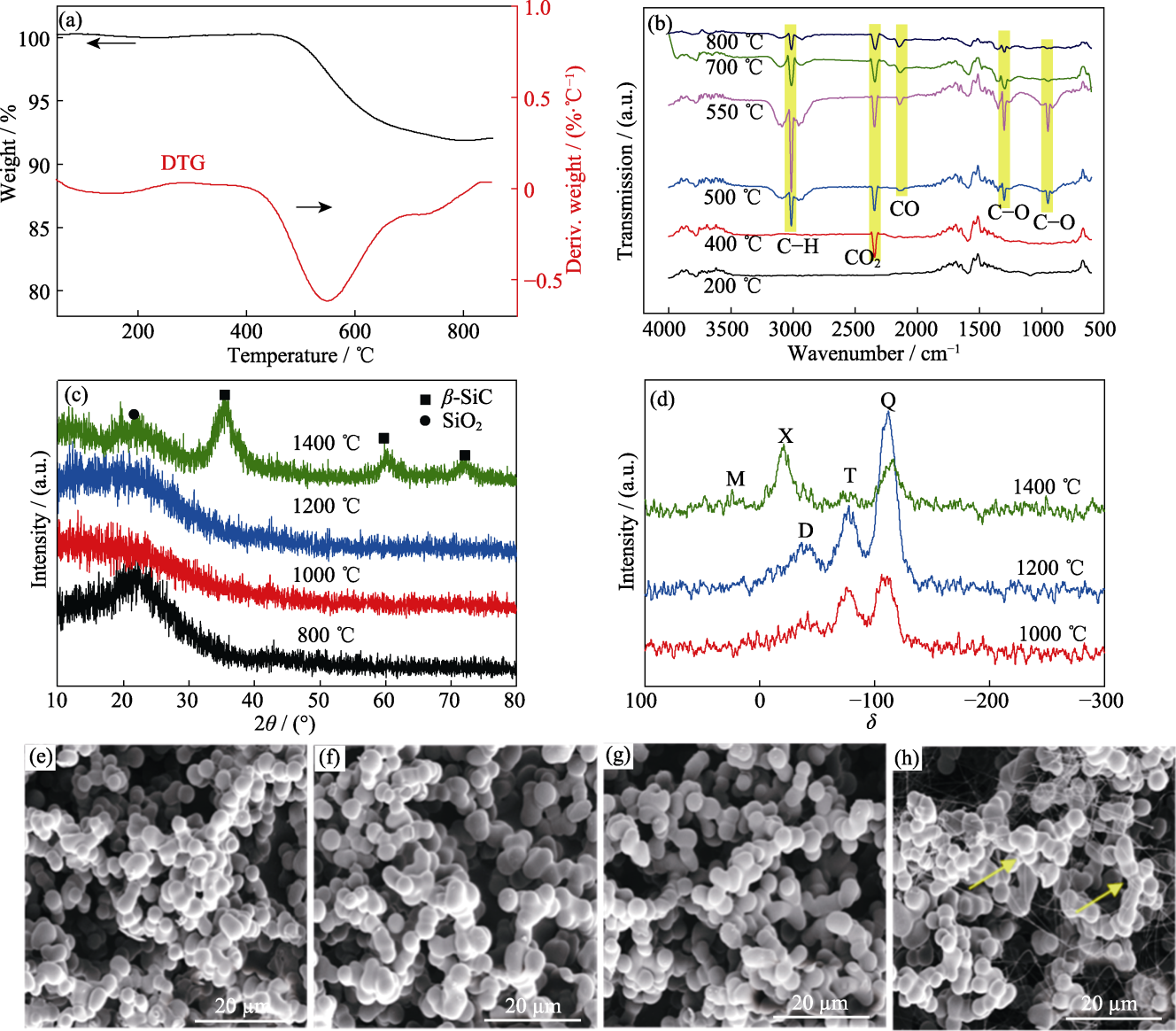
Fig. 4 (a, b) TG-IR spectra of sample V/VM-3 under N2 atmosphere; (c) XRD patterns, (d) NMR spectra and (e-h) SEM image of sample V/VM-3 after heat-treated at different temperatures (e) 800 ℃; (f) 1000 ℃; (g) 1200 ℃; (h) 1400 ℃
| [1] |
SONG J W, CHEN C J, YANG Z, et al. Highly compressible, anisotropic aerogel with aligned cellulose nanofibers. ACS Nano, 2018, 12(1): 140.
DOI PMID |
| [2] |
HAN X, HASSAN K T, HARVEY A, et al. Bioinspired synthesis of monolithic and layered aerogels. Advanced Materials, 2018, 30(23): 1706294.
DOI URL |
| [3] |
OU H H, YANG P J, LIN L H, et al. Carbon nitride aerogels for the photoredox conversion of water. Angewandte Chemie International Edition, 2017, 56(36): 10905-10910.
DOI URL |
| [4] |
CAI B, SAYEVICH V, GAPONIK N, et al. Emerging hierarchical aerogels: self-assembly of metal and semiconductor nanocrystals. Advanced Materials, 2018, 30(33): 1707518.
DOI URL |
| [5] |
KHALILY M A, EREN H, AKBAYRAK S, et al. Facile synthesis of three-dimensional Pt-TiO2 nano-networks: a highly active catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angewandte Chemie International Edition, 2016, 55(40): 12257-12261.
DOI URL |
| [6] |
HAGEDORN K, LI W, LIANG Q J, et al. Catalytically doped semiconductors for chemical gas sensing: aerogel-like aluminum- containing zinc oxide materials prepared in the gas phase. Advanced Functional Materials, 2016, 26(20): 3424-3437.
DOI URL |
| [7] |
NICOLA H, ULRICH S. Aerogels-airy materials: chemistry, structure, and properties. Angewandte Chemie International Edition, 1998, 37(1/2): 22-45.
DOI URL |
| [8] | BEAMISH J, HERMAN T. Adsorption and desorption of helium in aerogels. Physica B Condensed Matter, 2003, 329: 340-341. |
| [9] | BELLUNATO T, BRAEM A, BUZYKAEV A R, et al. Aerogel as cherenkov radiator for rich detectors. Nuclear Inst & Methods in Physics Research A, 2003, 502(1): 227-230. |
| [10] | KISTLER S S. Coherent expanded aerogel jellies. Nature, 1931, 127(3211): 741. |
| [11] |
DU A, ZHOU B, ZHANG Z, et al. A special material or new state of matter: a review and reconsideration of the aerogel. Materials, 2013, 6(3): 941-968.
DOI URL |
| [12] |
HE F, YU W J, FANG M H, et al. An overview on silica aerogels synthesized by siloxane co-precursors. Journal of Inorganic Materials, 2015, 30(12): 1243-1253.
DOI |
| [13] | NADARGI D Y, LATTHE S S, HIRASHIMA H, et al. Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous & Mesoporous Materials, 2009, 117(3): 617-626. |
| [14] |
KANAMORI K, AIZAWA M, NAKANISHI K, et al. New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties. Advanced Materials, 2007, 19(12): 1589-1593.
DOI URL |
| [15] | RAO A V, BHAGAT S D, HIRASHIMA H, et al. Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor. Journal of Colloid & Interface Science, 2006, 300(1): 279-285. |
| [16] |
ZU G Q, SHIMIZU T, KANAMORI K, et al. Transparent, superflexible doubly cross-linked polyvinylpolymethylsiloxane aerogel superinsulators via ambient pressure drying. ACS Nano, 2018, 12(1): 521-532.
DOI URL |
| [17] | ZU G Q, SHEN J, ZOU L P, et al. Preparation, mechanical properties and thermal properties of elastic aerogels. Journal of Inorganic Materials, 2014, 29(4): 417-422. |
| [18] |
HONG J Y, BAK B M, WIE J J, et al. Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions. Advanced Functional Materials, 2015, 25(7): 1053-1062.
DOI URL |
| [19] |
HAYASE G, KANAMORI K, HASEGAWA G, et al. A superamphiphobic macroporous silicone monolith with marshmallow- like flexibility. Angewandte Chemie International Edition, 2013, 52(41): 10788-10791.
DOI URL |
| [20] | QIU F X, ZHOU Y M, LIU J Z, et al. Study of 29Si MAS NMR spectroscopy and electro-optic property based on polyimide/SiO2. Photographic Science and Photochemistry, 2006, 24(1): 55-60. |
| [21] |
SHIMIZU T, KANAMORI K, MAENO A, et al. Transparent ethylene-bridged polymethylsiloxane aerogels and xerogels with improved bending flexibility. Langmuir, 2016, 32(50): 13427-13434.
PMID |
| [22] |
ZHANG Z, WANG X D, SHEN J. Effect of organic-inorganic crosslinking degree on the mechanical and thermal properties of aerogels. Journal of Inorganic Materials, 2020, 35(4): 454-460.
DOI |
| [23] |
ZU G Q, KANAMORI K, WANG X D, et al. Superelastic triple-network polyorganosiloxane-based aerogels as transparent thermal superinsulators and efficient separators. Chemistry of Materials, 2020, 32(4): 1595-1604.
DOI URL |
| [24] |
NAZERAN N, MOGHADDAS J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. Journal of Non-Crystalline Solids, 2017, 461: 1-11.
DOI URL |
| [25] | ZHANG Z, WANG X D, ZU G Q, et al. Resilient, fire-retardant and mechanically strong polyimide-polyvinylpolymethylsiloxane composite aerogel prepared via stepwise chemical liquid deposition. Materials & Design, 2019, 183: 108096. |
| [26] |
CHEN Z Q, CHEN Y F, LIU H B. Pyrolysis of phenolic resin by TG-MS and FTIR analysis. Advanced Materials Research, 2013, 631-632: 104-109.
DOI URL |
| [27] | 金晶, 徐晓秋, 杨雄发, 等. 聚硅氧烷热稳定性研究进展. 化工新型材料, 2010, 38(1): 17-19. |
| [28] | HUANG D M, GUO C N, ZHANG M Z, et al. Characteristics of nanoporous silica aerogel under high temperature from 950 ℃ to 1200 ℃. Materials & Design, 2017, 129: 82-90. |
| [29] | YANG G X, BISWAS P. Computer simulation of the aggregation and sintering restructuring of fractal-like clusters containing limited numbers of primary particles. Journal of Colloid & Interface Science, 1999, 211(1): 142-150. |
| [30] |
LI X K, LIU L, ZHANG Y X, et al. Synthesis of nanometre silicon carbide whiskers from binary carbonaceous silica aerogels. Carbon, 2001, 39(2): 159-165.
DOI URL |
| [31] |
MA J, YE F, LIN S J, et al. Large size and low density SiOC aerogel monolith prepared from triethoxyvinylsilane/tetraethoxysilane. Ceramics International, 2017, 43(7): 5774-5780.
DOI URL |
| [1] | YANG Mingkai, HUANG Zeai, ZHOU Yunxiao, LIU Tong, ZHANG Kuikui, TAN Hao, LIU Mengying, ZHAN Junjie, CHEN Guoxing, ZHOU Ying. Co-production of Few-layer Graphene and Hydrogen from Methane Pyrolysis Based on Cu and Metal Oxide-KCl Molten Medium [J]. Journal of Inorganic Materials, 2025, 40(5): 473-480. |
| [2] | WANG Wenting, XU Jingjun, MA Ke, LI Meishuan, LI Xingchao, LI Tongqi. Oxidation Behavior at 1000-1300 ℃ in air of Ti2AlC-20TiB2 Synthesized by in-situ Reaction/Hot Pressing [J]. Journal of Inorganic Materials, 2025, 40(1): 31-38. |
| [3] | QUAN Wenxin, YU Yiping, FANG Bing, LI Wei, WANG Song. Oxidation Behavior and Meso-macro Model of Tubular C/SiC Composites in High-temperature Environment [J]. Journal of Inorganic Materials, 2024, 39(8): 920-928. |
| [4] | MAO Aiqin, LU Wenyu, JIA Yanggang, WANG Ranran, SUN Jing. Flexible Piezoelectric Devices and Their Wearable Applications [J]. Journal of Inorganic Materials, 2023, 38(7): 717-730. |
| [5] | GU Xuesu, YIN Jie, WANG Kanglong, CUI Chong, MEI Hui, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Effect of Particle Grading on Properties of Silicon Carbide Ceramics by Binder Jetting Printing [J]. Journal of Inorganic Materials, 2023, 38(12): 1373-1378. |
| [6] | CAI Jia, ZHAO Fangxia, FAN Dong, HUANG Liping, NIU Yaran, ZHENG Xuebin, ZHANG Zhenzhong. Pyrolysis Behavior and Laser Ablation Resistance of PCS in Polycarbosilane Composite Coatings [J]. Journal of Inorganic Materials, 2023, 38(11): 1271-1280. |
| [7] | LEI Yiming, ZHANG Jie, BAI Guanghai, ZHANG Yanwei, WANG Xiaohui, WANG Jingyang. Influence of Al Content on Oxidation Resistance of Phase-pure Ti2AlC under Simulated Loss-of-coolant Accident Conditions [J]. Journal of Inorganic Materials, 2021, 36(10): 1097-1102. |
| [8] | DU Juan, LIU Lei, YU Yifeng, ZHANG Yue, LÜ Haijun, CHEN Aibing. Hollow Carbon Sphere with Tunable Structure by Encapsulation Pyrolysis Synchronous Deposition for Cefalexin Adsorption [J]. Journal of Inorganic Materials, 2020, 35(5): 608-616. |
| [9] | LI Xuqin, TAN Zhiyong, CHENG Laifei, ZHOU Lingke, GAO Jian. Tensile Behaviors and Matrix Cracking Mechanism of C/SiCN Composite Prepared by Precursor Infiltration Pyrolysis Method [J]. Journal of Inorganic Materials, 2020, 35(11): 1227-1233. |
| [10] | ZHANG Cheng, GONG Jun-Jie, DONG Zhi-Jun, MENG Jian, ZHOU Si-Cheng, YUAN Guan-Ming, LI Xuan-Ke. HfC Precursor: Synthesis and Pyrolysis Behavior [J]. Journal of Inorganic Materials, 2017, 32(10): 1095-1101. |
| [11] | XU Shun-Jian, LUO Yu-Feng, ZHONG Wei, XIAO Zong-Hu, LOU Yong-Ping, OU Hui. Quasi-solid-state Dye-sensitized Solar Cells Employing Fibers Stacked Paper Carbons as Efficient Counter Electrodes [J]. Journal of Inorganic Materials, 2015, 30(1): 29-34. |
| [12] | CHEN Ming-Zhe, GUO Xiao-Dong, ZHONG Ben-He, YAN Hui-Min, ZHANG Ji-Bin, LIU Ju. High Energy Density Spinel LiCr0.2Ni0.4Mn1.4O4 Cathode Material Prepared by Spray Pyrolysis Method [J]. Journal of Inorganic Materials, 2014, 29(9): 917-923. |
| [13] | XU Run, LI Xin-Da, LI Zhi-Qiang, ZHAO Ren-Yu, FAN Gen-Lian, XIONG Ding-Bang, TANG Jie, XU Yong, ZHANG Di. Preparation and Reaction Kinetics of Carbon Nanotubes/Aluminum Composite Powders Using Polymer Pyrolysis Method [J]. Journal of Inorganic Materials, 2014, 29(7): 687-694. |
| [14] | CHEN Wei, LIU Yang-Qiao, LUO Jian-Qiang, JIN Xi-Hai, SUN Jing, GAO Lian. Fabrications of TiO2Photoanodes for Flexible Dye-sensitized Solar Cells [J]. Journal of Inorganic Materials, 2014, 29(6): 561-570. |
| [15] | YU Jiao-Zhu, LI Lin, JIN Xin, DING Ling-Hua, WANG Tong-Hua. Preparation of Organic/Inorganic Membrane by PDMS Low-temperature Pyrolysis [J]. Journal of Inorganic Materials, 2014, 29(2): 137-142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||