
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (5): 541-548.DOI: 10.15541/jim20190281
Special Issue: 2020年环境材料论文精选(三)有机小分子去除; 优秀作者论文集锦; 2019~2020年度优秀作者作品欣赏:环境材料
Previous Articles Next Articles
WU Fan1,ZHAO Ziyan1,LI Bangxin1,DONG Fan2,ZHOU Ying1( )
)
Received:2019-06-10
Revised:2019-10-02
Published:2020-05-20
Online:2019-12-04
Supported by:CLC Number:
WU Fan, ZHAO Ziyan, LI Bangxin, DONG Fan, ZHOU Ying. Interfacial Oxygen Vacancy of Bi2O2CO3/PPy and its Visible-light Photocatalytic NO Oxidation Mechanism[J]. Journal of Inorganic Materials, 2020, 35(5): 541-548.
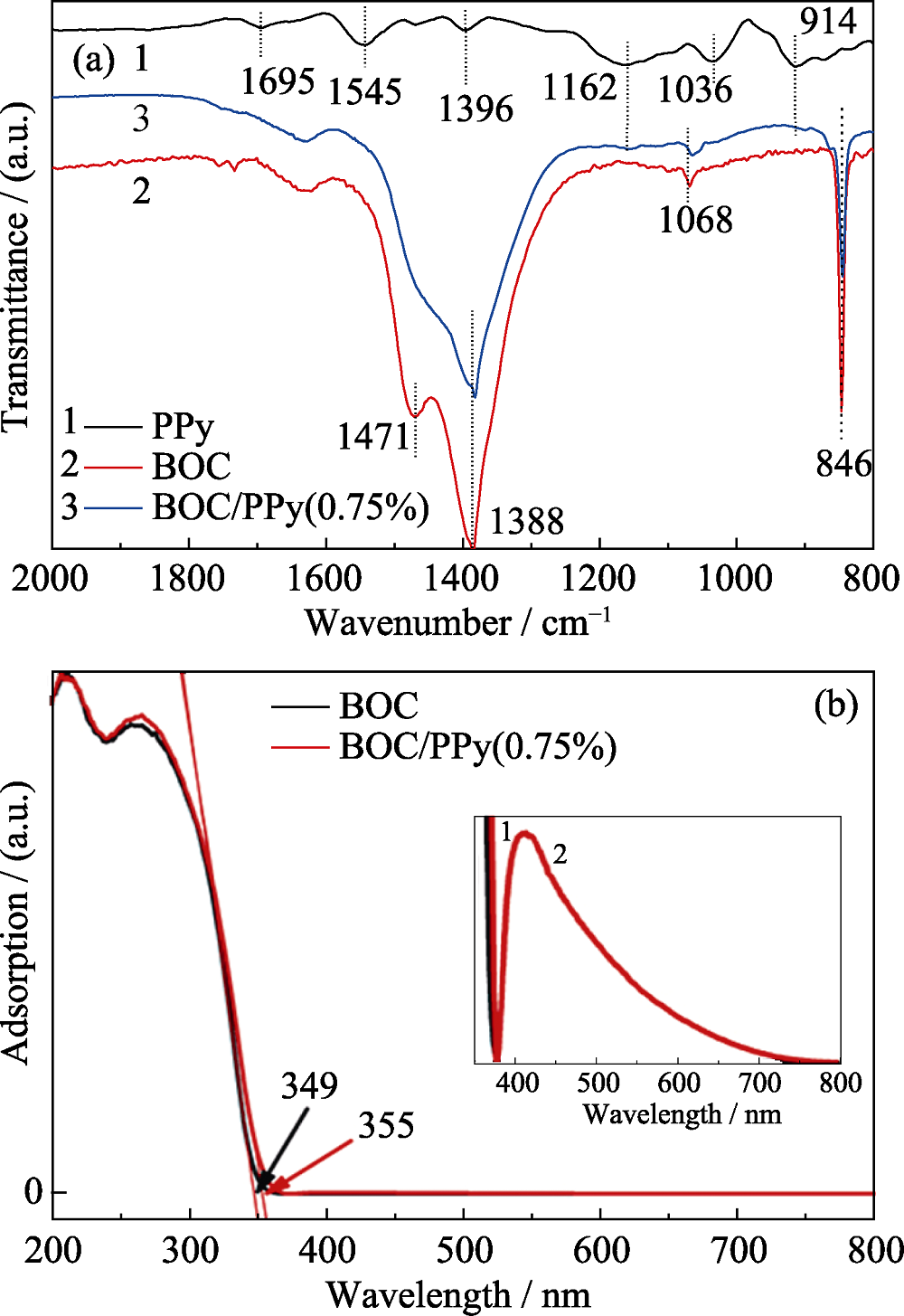
Fig. 2 (a) DRIFTS spectra of PPy, BOC and BOC/PPy(0.75%); (b) UV-Vis spectra and of BOC and BOC/PPy(0.75%) with insert showing the enlarged spectra from 350 nm to 800 nm
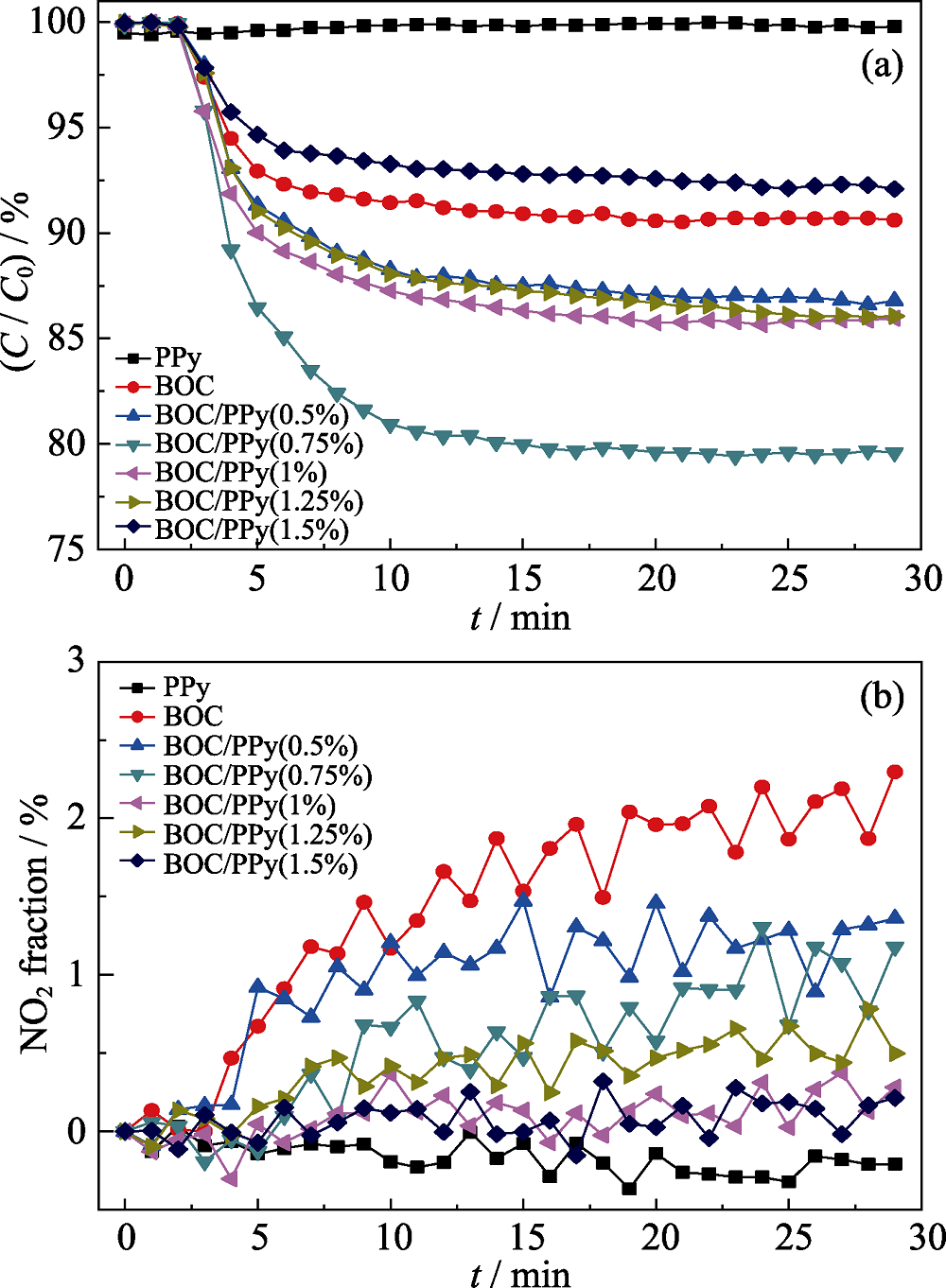
Fig. 7 NO oxidation of BOC and BOC/PPy(0.75%) composites under visible light irradiation (>420 nm) (a) Photocatalytic removal ratio of NO; (b) Generation of NO2
| Wavenumber/cm-1 | Assignment | Ref. |
|---|---|---|
| 1262 | Monodentate nitrate | [38] |
| 1248 | Bridge nitrate | [39] |
| 1183 | Nitrite | [40] |
| 1159 | NO- | [40] |
| 1091 | Nitrite | [41] |
| 1032, 1010 | Bidentate nitrate | [38, 40] |
| 984 | Bridge nitrate | [42] |
Table 1 Assignments of adsorption peaks in IR spectra during NO adsorption and photocatalytic oxidation
| Wavenumber/cm-1 | Assignment | Ref. |
|---|---|---|
| 1262 | Monodentate nitrate | [38] |
| 1248 | Bridge nitrate | [39] |
| 1183 | Nitrite | [40] |
| 1159 | NO- | [40] |
| 1091 | Nitrite | [41] |
| 1032, 1010 | Bidentate nitrate | [38, 40] |
| 984 | Bridge nitrate | [42] |
| [1] |
AI Z H, HO W, LEE S , et al. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environmental Science Technology, 2009,43:4143-4150.
DOI URL PMID |
| [2] |
WANG HUA, SU YAN, ZHAO HUA XIN , et al. Photocatalytic oxidation of aqueous ammonia using atomic single layer graphitic- C3N4. Environmental Science and Technology, 2014,48:11984-11990.
DOI URL PMID |
| [3] | LASEK J, YU YI-HUI, WU J C S . Removal of NO x by photocatalytic processes. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2013,14:29-52. |
| [4] |
ASAHI R, MORIKAWA T, IRIE H , et al. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: designs, developments, and prospects. Chemical Reviews, 2014,114(19):9824-9852.
DOI URL PMID |
| [5] |
KAPILASHRAMI M, ZHANG YAN-FENG, LIU YI-SHENG , et al. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chemical Reviews, 2014,114(19):9662-9707.
DOI URL PMID |
| [6] |
LIU YANG, YU SHAN, ZHENG KAI-WEN , et al. NO photo- oxidation and in-situ DRIFTS studies on N-doped Bi2O2CO3/CdSe quantum dot composite. Journal of Inorganic Materials, 2019,34(4):425-432.
DOI URL |
| [7] | NI ZI-LIN, SUN YAN-JUAN, ZHANG YU-XIN , et al. Fabrication, modification and application of (BiO)2CO3-based photocatalysts: a review. Applied Surface Science, 2016,365:314-335. |
| [8] | LIU YUAN-YUAN, WANG ZE-YAN, HUANG BAI-BIAO , et al. Preparation, electronic structure, and photocatalytic properties of Bi2O2CO3 nanosheet. Applied Surface Science, 2010,257(1):172-175. |
| [9] |
NAKAMURA I, NEGISHI N, KUTSUNA S , et al. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. Journal of Molecular Catalysis A: Chemical, 2000,161(1):205-212.
DOI URL |
| [10] |
LAI KANG-RONG, WEI WEI, ZHU YING-TAO , et al. Effects of oxygen vacancy and N-doping on the electronic and photocatalytic properties of Bi2MO6( M=Mo, W). Journal of Solid State Chemistry, 2012,187(3):103-108.
DOI URL |
| [11] | BILMES S A, MANDELBAUM P . Surface and electronic structure of titanium dioxide photocatalysts. Journal of Physical Chemistry B, 2000,104(42):9851-9858. |
| [12] |
JING LI-QIANG, XIN BAI-FU, YUAN FU-LONG , et al. Effects of surface oxygen vacancies on photophysical and photochemical processes of Zn-doped TiO2 nanoparticles and their relationships. Journal of Physical Chemistry B, 2006,110(36):17860-17865.
DOI URL PMID |
| [13] |
NOWPTNY J . Titanium dioxide-based semiconductors for solar- driven environmentally friendly applications: impact of point defects on performance. Energy Environmental Science, 2008,1(5):565-572.
DOI URL |
| [14] |
NOWOTNY M K, SHEPPARD L R, BAK T , et al. Defect chemistry of titanium dioxide. application of defect engineering in processing of TiO2-based photocatalysts. Journal of Physical Chemistry C, 2008,112(14):5275-5300.
DOI URL |
| [15] |
WANG JIAN-CHUN, LIU PING, FU XIAN-ZHI , et al. Relationship between oxygen defects and the photocatalytic property of ZnO nanocrystals in nafion membranes. Langmuir, 2009,25(2):1218-1223.
DOI URL PMID |
| [16] |
SCHAUB R, THOSTRUP P, LOPEZ , et al. Oxygen vacancies as active sites for water dissociation on rutile TiO2( 110). Physical Review Letters, 2001,87(26):266104-266107.
DOI URL PMID |
| [17] |
CHEN XIAO-BO, LIU LEI, YU P Y , et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science, 2011,331(6018):746-750.
DOI URL PMID |
| [18] |
ERIKSEN S, EGDELL R G . Electronic excitations at oxygen deficient TiO2(110) surfaces: a study by EELS. Surface Science, 1987,180(1):263-278.
DOI URL |
| [19] | KNOTEK M L, FEIBELMAN P J . Ion desorption by core-hole auger decay. Physical Review Letters, 1978,14(40):964-967. |
| [20] | PANAYOTOV D A, MORRIS J R . Thermal decomposition of a chemical warfare agent simulant (DMMP) on TiO2: adsorbate reactions with lattice oxygen as studied by infrared spectroscopy. Journal of Physical Chemistry C, 2009,113(35):15684-15691. |
| [21] |
WACHS I E, JEHNG J M, UEDA W . Determination of the chemical nature of active surface sites present on bulk mixed metal oxide catalysts. Journal of Physical Chemistry, 2005,109(6):2275-2284.
DOI URL PMID |
| [22] |
ZHAO ZI-YAN, ZHOU YING, WANG FANG , et al. Polyaniline- decorated {001} facets of Bi2O2CO3 nanosheets: in situ oxygen vacancy formation and enhanced visible light photocatalytic activity. ACS Applied Materials Interfaces, 2015,7:730-737.
DOI URL PMID |
| [23] |
ZHAO WEI, WANG YUN, WANG AI JIAN , et al. Novel Bi2O2CO3/polypyrrole/g-C3N4 nanocomposites with efficient photocatalytic and nonlinear optical properties. RSC Advances, 2017,7(13):7658-7670.
DOI URL |
| [24] | ULLAH H, TAHIR A A, MALLICKT K . Polypyrrole/TiO2 composites for the application of photocatalysis. Sensors and Actuators B, 2017,241:1161-1169. |
| [25] | MOHD TARMIZI E Z, BAQIAH H, TALIB Z A . Facile synthesis and characterizations of polypyrrole/BiOCl hybrid composites. Journal of Solid State Electrochemistry, 2017,21(11):3247-3255. |
| [26] |
ZHAO ZI-YAN, CAO YUE-HAN, DONG FAN , et al. The activation of oxygen through oxygen vacancy on BiOCl/PPy to inhibit toxic intermediates and enhance the activity of photocatalytic NO removal. Nanoscale, 2019,11:6360-6367.
DOI URL PMID |
| [27] | ZHANG QIAN, ZHOU YING, WANG FANG , et al. From semiconductors to semimetals: bismuth as photocatalyst for NO oxidation in air. Journal of Materials Chemistry A, 2014,2:11065-11072. |
| [28] | CHEN XUE FANG, HUANG YING, ZHANG KAI CHUANG , et al. Synthesis and high-performance of carbonaceous polypyrrole nanotubes coated with SnS2 nanosheets anode materials for lithium ion batteries. Chemical Engineering Journal, 2017,330:470-479. |
| [29] | LIU YI-XIN, MA HONG-MA, ZHANG YONG , et al. Visible light photoelectrochemical aptasensor for adenosine detection based on CdS/PPy/g-C3N4 nanocomposites. Biosens and Bioelectronics, 2016,86:439-445. |
| [30] |
DONG FAN, ZHENG AN-MIN, SUN YAN-JUAN , et al. One-pot template-free synthesis, growth mechanism and enhanced photocatalytic activity of monodisperse (BiO)2CO3 hierarchical hollow microspheres self-assembled with single-crystalline nanosheets. CrystEngComm, 2012,14:3534-3544.
DOI URL |
| [31] |
DENG FAN, MIN LU-JUAN, LUO XU-BIAO , et al. Visible-light photocatalytic degradation performances and thermal stability due to the synergetic effect of TiO2 with conductive copolymers of polyaniline and polypyrrole. Nanoscale, 2013,5:8703-8710.
DOI URL PMID |
| [32] | MADHUSUDAN P, RAN JING-RAN, ZHANG JUN , et al. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Applied Catalysis B: Environmental, 2011,110:286-295. |
| [33] |
DONG FAN, SUN YAN-JUAN, FU MIN , et al. Novel in situ N-Doped (BiO)2CO3 hierarchical microspheres self-assembled by nanosheets as efficient and durable visible light driven photocatalyst. Langmuir, 2012,28:766-773.
DOI URL PMID |
| [34] |
YE LI-QUN, DENG KE-JIAN, XU FENG , et al. Increasing visible- light absorption for photocatalysis with black BiOCl. Physical Chemistry Chemical Physics, 2012,14:82-85.
DOI URL PMID |
| [35] |
PAN XIAO-YANG, YANG MIN-QUAN, FU XIAN-ZHI , et al. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale, 2013,5:3601-3614.
DOI URL PMID |
| [36] |
LEI FENG-CAI, SUN YONG-FU, LIU KA-TONG , et al. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. Journal of American Chemical Society, 2014,136:6826-6829.
DOI URL PMID |
| [37] | ZHOU YING, ZHANG QIAN, LIN YUAN-HUA , et al. One-step hydrothermal synthesis of hierarchical Ag/Bi2WO6 composites: in situ growth monitoring and photocatalytic activity studies. Science China Chemistry, 2013,56:435-442. |
| [38] | TANG NIAN, LIU YUE, WANG HAI-QIANG , et al. Mechanism study of NO catalytic oxidation over MnOx/TiO2 catalysts. Journal of Physical of Chemistry C, 2011,115:8214-8220. |
| [39] |
KANTCHEVA M . Identification, stability, and reactivity of NO x species adsorbed on titania-supported manganese catalysts. Journal of Catalysis, 2001,204:479-494.
DOI URL |
| [40] |
HADJIIVANOV K, AVREYSKA V, KLISSURSKI D , et al. Surface species formed after NO adsorption and NO+O2 coadsorption on ZrO2 and Sulfated ZrO2: an FTIR spectroscopic study. Langmuir, 2002,18:1619-1625.
DOI URL |
| [41] | LAANE J, OHLSEN J R . Characterization of Nitrogen Oxides by Vibrational Spectroscopy. John Wiley & Sons, Inc. 1980,27:465-513. |
| [42] | CHEN MEI, WANG ZHI-HUA, HAN DONG-MEI , et al. Porous ZnO polygonal nanoflakes: synthesis, use in high-sensitivity NO2 gas sensor, and proposed mechanism of gas sensing. Journal of Physical Chemistry C, 2011,115(26):12763-12773. |
| [43] | LIU YANG, YU SHAN, ZHAO ZI-YANG , et al. N-doped Bi2O2CO3/graphene quantum dot composite photocatalyst: enhanced visible-light photocatalytic NO oxidation and in situ DRIFTS studies. Journal of Physical Chemistry C, 2017,121:12168-12177. |
| [44] | LI CAI, JIANG HUI, WANG LU-XI . Enhanced photo-stability and photocatalytic activity of Ag3PO4 via modification with BiPO4 and polypyrrole. Applied Surface Science, 2017,420:43-52. |
| [45] |
WANG ZHEN-YU, GUAN WEI, SUN YAN-JUAN , et al. Water- assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale, 2015,7(6):2471-2479.
DOI URL PMID |
| [1] | FAN Xiaoxuan, ZHENG Yonggui, XU Lirong, YAO Zimin, CAO Shuo, WANG Kexin, WANG Jiwei. Organic Pollutant Fenton Degradation Driven by Self-activated Afterglow from Oxygen-vacancy-rich LiYScGeO4: Bi3+ Long Afterglow Phosphor [J]. Journal of Inorganic Materials, 2025, 40(5): 481-488. |
| [2] | JIA Xianghua, ZHANG Huixia, LIU Yanfeng, ZUO Guihong. Cu2O/Cu Hollow Spherical Heterojunction Photocatalysts Prepared by Wet Chemical Approach [J]. Journal of Inorganic Materials, 2025, 40(4): 397-404. |
| [3] | MA Binbin, ZHONG Wanling, HAN Jian, CHEN Liangyu, SUN Jingjing, LEI Caixia. ZIF-8/TiO2 Composite Mesocrystals: Preparation and Photocatalytic Activity [J]. Journal of Inorganic Materials, 2024, 39(8): 937-944. |
| [4] | CAO Qingqing, CHEN Xiangyu, WU Jianhao, WANG Xiaozhuo, WANG Yixuan, WANG Yuhan, LI Chunyan, RU Fei, LI Lan, CHEN Zhi. Visible-light Photodegradation of Tetracycline Hydrochloride on Self-sensitive Carbon-nitride Microspheres Enhanced by SiO2 [J]. Journal of Inorganic Materials, 2024, 39(7): 787-792. |
| [5] | WANG Zhaoyang, QIN Peng, JIANG Yin, FENG Xiaobo, YANG Peizhi, HUANG Fuqiang. Sandwich Structured Ru@TiO2 Composite for Efficient Photocatalytic Tetracycline Degradation [J]. Journal of Inorganic Materials, 2024, 39(4): 383-389. |
| [6] | DING Ningning, SUN Jianhua, WEI Xu, SUN Lixia. Monitoring Ammonia at Room Temperature of p-Aminobenzene Sulfonic Acid Modified MoO3/PPy Composites [J]. Journal of Inorganic Materials, 2024, 39(11): 1245-1253. |
| [7] | WU Lin, HU Minglei, WANG Liping, HUANG Shaomeng, ZHOU Xiangyuan. Preparation of TiHAP@g-C3N4 Heterojunction and Photocatalytic Degradation of Methyl Orange [J]. Journal of Inorganic Materials, 2023, 38(5): 503-510. |
| [8] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [9] | SUN Chen, ZHAO Kunfeng, YI Zhiguo. Research Progress in Catalytic Total Oxidation of Methane [J]. Journal of Inorganic Materials, 2023, 38(11): 1245-1256. |
| [10] | JIA Xin, LI Jinyu, DING Shihao, SHEN Qianqian, JIA Husheng, XUE Jinbo. Synergy Effect of Pd Nanoparticles and Oxygen Vacancies for Enhancing TiO2 Photocatalytic CO2 Reduction [J]. Journal of Inorganic Materials, 2023, 38(11): 1301-1308. |
| [11] | MA Xinquan, LI Xibao, CHEN Zhi, FENG Zhijun, HUANG Juntong. BiOBr/ZnMoO4 Step-scheme Heterojunction: Construction and Photocatalytic Degradation Properties [J]. Journal of Inorganic Materials, 2023, 38(1): 62-70. |
| [12] | CHEN Hanxiang, ZHOU Min, MO Zhao, YI Jianjian, LI Huaming, XU Hui. 0D/2D CoN/g-C3N4 Composites: Structure and Photocatalytic Performance for Hydrogen Production [J]. Journal of Inorganic Materials, 2022, 37(9): 1001-1008. |
| [13] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [14] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [15] | WANG Xiaojun, XU Wen, LIU Runlu, PAN Hui, ZHU Shenmin. Preparation and Properties of Ag@C3N4 Photocatalyst Supported by Hydrogel [J]. Journal of Inorganic Materials, 2022, 37(7): 731-740. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||