
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (11): 1242-1248.DOI: 10.15541/jim20160121
• Orginal Article • Previous Articles Next Articles
YAN Hui1, Qi Lu2, ZHANG Ding3, WANG Zheng-De1, LIU Yun-Ying1, WANG Xiao-Xia1, ZHU Tie-Yong4
Received:2016-03-07
Revised:2016-04-08
Published:2016-11-10
Online:2016-10-25
Supported by:CLC Number:
YAN Hui, Qi Lu, ZHANG Ding, WANG Zheng-De, LIU Yun-Ying, WANG Xiao-Xia, ZHU Tie-Yong. Hydrothermal Synthesis and Electrochemical Performance of Spherical Li4Ti5O12 as Anode Material for Lithium-ion Secondary Battery[J]. Journal of Inorganic Materials, 2016, 31(11): 1242-1248.
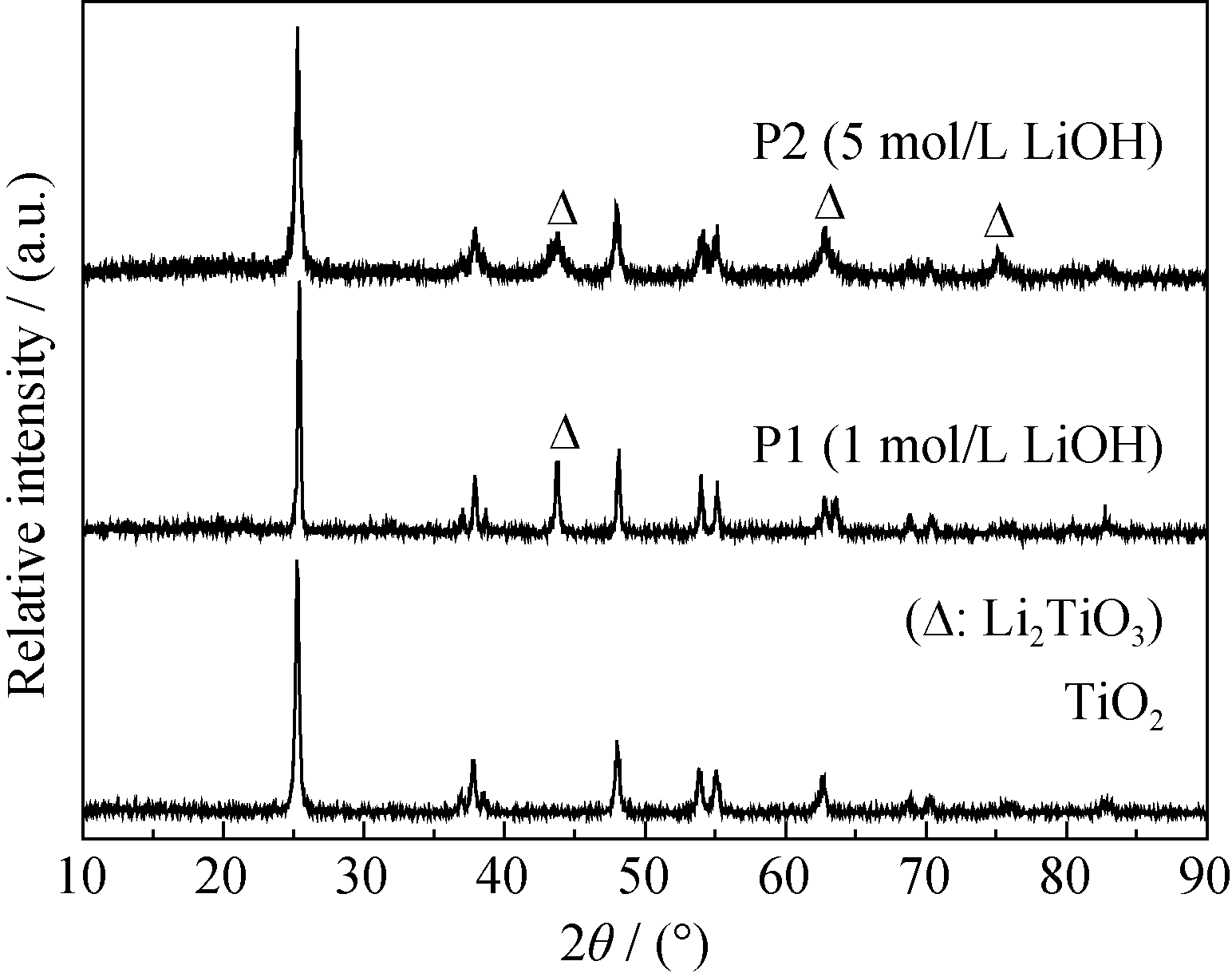
Fig. 2 XRD pattern of the precursors obtained from the hydrothermal reaction between TiO2 and LiOH solution with different concentrations (1 mol/L and 5 mol/L)
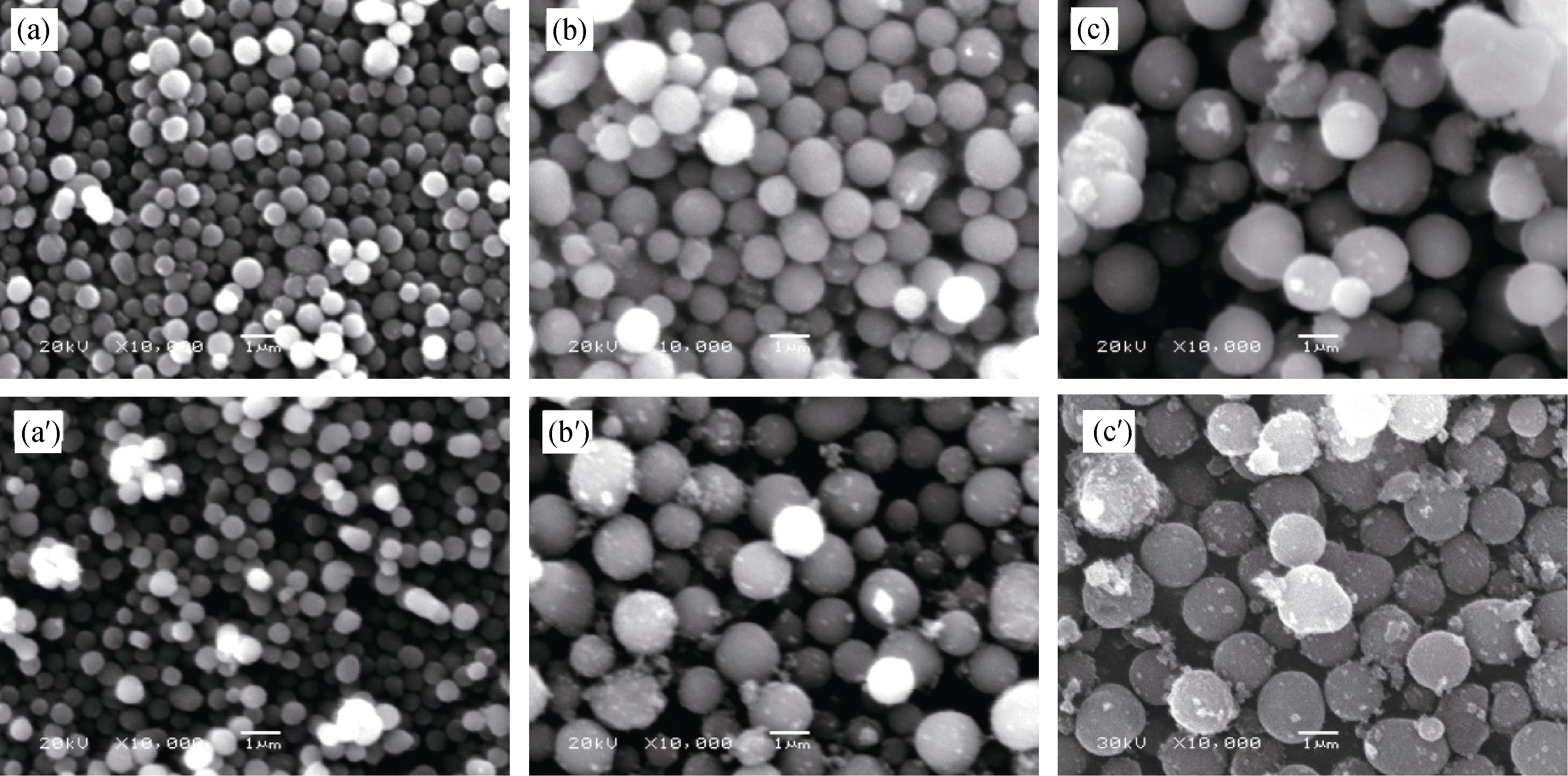
Fig. 5 SEM images of precursors (a′-c′) after hydrothermal reaction and the final spherical Li4Ti5O12 (a-c) samples with different particle sizes (a) 0.5 µm; (b) 1.0 µm; (c) 1.5 µm
| [1] | 其鲁. 电动汽车用锂离子二次电池, 2版. 北京: 科学出版社, 2010: 118-162. |
| [2] | AN P, QI L, Applications and Development of Li- ion Secondary Batteries.Acta Scientiarum Naturalium Universitatis Pekinensis, 2006, 42(sup): 1-7. |
| [3] | LI W, TONG G L G, MU Q Y, et al. Chemical pattern recognition applied to the analysis of effect of technology factors on the properties of Li-Mn-O spinel oxide.Acta Physico-Chimica Sinica, 2007, 23(sup): 10-13. |
| [4] | OHZUKU T, UEDA A, YAMAMOTO N.Zero-strain insertion material of Li[Li1/3Ti4/3]O4 for rechargeable lithium cells.Journal of the Electrochemical Society, 1995, 142(5): 1431-1435. |
| [5] | WINTER M, BESENHARD J O, SPAHR M E, et al.Insertion electrode materials for rechargeable lithium batteries.Advanced Materials, 1998, 10(10): 725-763. |
| [6] | TARASCON J M, ARMAND M.Issues and challenges facing rechargeable lithium batteries.Nature, 2001, 414(6861): 359-367. |
| [7] | ZAGHIB K, SIMONEAU M, ARMAND M, et al. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. Journal of Power Sources, 1999, 81-82: 300-305. |
| [8] | ABRAHAM K M, PASQUARIELLO D M, WILLSTAEDT E B.Preparation and characterization of some lithium insertion anodes for secondary lithium batteries.Journal of the Electrochemical Society, 1990, 137(3): 743-749. |
| [9] | POIZOT P, LARUELLE S, GRUGEON S, et al.Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries.Nature, 2000, 407(6803): 496-499. |
| [10] | TAKEDA Y, NISHIJIMA M, YAMAHATA M, et al.Lithium secondary batteries using a lithium cobalt nitride, Li2.6Co0.4N, as the anode.Solid State Ionics, 2000, 130(1/2): 61-69. |
| [11] | XIA Y Y, SAKAI T, FUJIEDA T, et al.Flake Cu-Sn alloys as negative electrode materials for rechargeable lithium batteries.Journal of the Electrochemical Society, 2001, 148(5): A471-A481. |
| [12] | KITAURA H, HAYASHI A, TADANAGA K, et al.High-rate performance of all-solid-state lithium secondary batteries using Li4Ti5O12 electrode.Journal of Power Sources, 2009, 189(1): 145-148. |
| [13] | YUAN T, CAI R, WANG K, et al.Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery.Ceramics International, 2009, 35(5): 1757-1768. |
| [14] | NAOI K, ISHIMOTO S, ISOBE Y, et al.High-rate nano-crystalline Li4Ti5O12 attached on carbon nano-fibers for hybrid supercapacitors.Journal of Power Sources, 2010, 195(18): 6250-6254. |
| [15] | ALLEN J L, JOW T R, WOLFENSTINE J.Low temperature performance of nanophase Li4Ti5O12.Journal of Power Sources, 2006, 159(2): 1340-1345. |
| [16] | ZHANG Y L, HU X B, XU Y L, et al.Recent progress of Li4Ti5O12 with different morphologies as anode material.Acta Chimica Sinica, 2013, 71(10): 1341-1353. |
| [17] | HUANG S H, WEN Z Y, ZHU X J, et al.Effects of dopant on the electrochemical performance of Li4Ti5O12 as electrode material for lithium ion batteries.Journal of Power Sources, 2007, 165(1): 408-412. |
| [18] | HUANG S H, WEN Z Y, ZHANG J C, et al.Li4Ti5O12/Ag composite as electrode materials for lithium-ion battery.Solid State Ionics, 2006, 177(9/10): 851-855. |
| [19] | HUANG S H, WEN Z Y, GU Z H, et al.Preparation and cycling performance of Al3+ and F- co-substituted compounds Li4AlxTi5-xFyO12-y.Electrochimica Acta, 2005, 50(20): 4057-4062. |
| [20] | GUAN X F, CHEN X M, LI G S.Direct synthesis of carbon-coated Li4Ti5O12 mesoporous nanoparticles for high-rate lithium-ion batteries.RSC Advances, 2013, 3(9): 3088-3094. |
| [21] | SHEN L F, YUAN C Z, LUO H J, et al.In situ synthesis of high-loading Li4Ti5O12-graphene hybrid nanostructures for high rate lithium ion batteries.Nanoscale, 2011, 3(2): 572-574. |
| [22] | NUGROHO A, CHANG W, KIM S J, et al.Superior high rate performance of core-shell Li4Ti5O12/carbon nanocomposite synthesized by a supercritical alcohol approach.RSC Advances, 2012, 2(29): 10805-10808. |
| [23] | WU F X, WANG Z X, LI X H, et al.Characterization of spherical-shaped Li4Ti5O12 prepared by spray drying.Electrochimica Acta, 2012, 78: 331-339. |
| [24] | WEN S J, LI G J, REN R M, et al.Preparation of spherical Li4Ti5O12 anode materials by spray drying.Materials Letters, 2015, 148: 130-133. |
| [25] | WANG J, CHENG X L, WANG Z G, et al.Synthesis and electrochemical properties of highly dispersed Li4Ti5O12 nano crystalline as anode material for lithium secondary batteries.Journal of Inorganic Materials, 2010, 25(3): 235-241. |
| [26] | HE Z J, WANG Z X, WU F X, et al.Spherical Li4Ti5O12 synthesized by spray drying from a different kind of solution.Journal of Alloys and Compounds, 2012, 540: 39-45. |
| [27] | GAO J, JIANG C Y, YING J R, et al.Preparation and characterization of high-density spherical Li4Ti5O12 anode material for lithium secondary batteries.Journal of Power Sources, 2006, 155(2): 364-367. |
| [28] | DU G J, LIU Z L, TAY S W, et al.Mesoporous spherical Li4Ti5O12 as high-performance anodes for lithium-ion batteries.Chemistry-An Asian Journal, 2014, 9(9): 2514-2518. |
| [29] | ARICO A S, BRUCE P, SCROSATI B, et al.Nanostructured materials for advanced energy conversion and storage devices.Nature Materials, 2005, 4(5): 366-377. |
| [30] | AMINE K, BELHAROUAK I, CHEN Z, et al.Nanostructured anode material for high-power battery system in electric vehicles.Advanced Materials, 2010, 22(28): 3052-3057. |
| [31] | YAN H, ZHANG H, ZHANG D, et al.Hydrothermal synthesis of spherical Li4Ti5O12 as anode material for high power lithium-ion secondary battery.Acta Physico-Chimica Sinica, 2011, 27(9): 2118-2122. |
| [32] | YAN H, ZHU Z, ZHANG D, et al.A new hydrothermal synthesis of spherical Li4Ti5O12 anode material for lithium-ion secondary batteries.Journal of Power Sources, 2012, 219: 45-51. |
| [33] | FATTAKHOVA D, PETRYKIN V, BRUS J, et al.Solvothermal synthesis and electrochemical behavior of nanocrystalline cubic Li-Ti-O oxides with cationic disorder.Solid State Ionics, 2005, 176(23/24): 1877-1885. |
| [34] | FATTAKHOVA D, KRTIL P.Electrochemical activity of hydrothermally synthesized Li-Ti-O cubic oxides toward Li insertion.Journal of The Electrochemical Society, 2002, 149(9): A1224-A1229. |
| [35] | JIANG C H, HOSONO E, ICHIHARA M, et al.Synthesis of nanocrystalline Li4Ti5O12 by chemical lithiation of anatase nanocrystals and post annealing.Journal of The Electrochemical Society, 2008, 155(8): A553-A556. |
| [36] | LAI C, DOU Y Y, LI X, et al.Improvement of the high rate capability of hierarchical structured Li4Ti5O12 induced by the pseudocapacitive effect.Journal of Power Sources, 2010, 195(11): 3676-3679. |
| [37] | RAHMAN M M, WANG J Z, HASSAN M F, et al.Basic molten salt process-A new route for synthesis of nanocrystalline Li4Ti5O12-TiO2 anode material for Li-ion batteries using eutectic mixture of LiNO3-LiOH-Li2O2.Journal of Power Sources, 2010, 195(13): 4297-4303. |
| [38] | BRUCE P G, SCROSATI B, TARASCON J M.Nanomaterials for rechargeable lithium batteries.Angewandte Chemie-Interna tional Edition, 2008, 47(16): 2930-2946. |
| [1] | FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders [J]. Journal of Inorganic Materials, 2025, 40(1): 104-110. |
| [2] | LIU Yan, QIN Xianpeng, GAN Lin, ZHOU Guohong, ZHANG Tianjin, WANG Shiwei, CHEN Hetuo. Preparation of Sub-micron Spherical Y2O3 Particles and Transparent Ceramics [J]. Journal of Inorganic Materials, 2024, 39(6): 691-696. |
| [3] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [4] | SONG Keke, HUANG Hao, LU Mengjie, YANG Anchun, WENG Jie, DUAN Ke. Hydrothermal Preparation and Characterization of Zn, Si, Mg, Fe Doped Hydroxyapatite [J]. Journal of Inorganic Materials, 2021, 36(10): 1091-1096. |
| [5] | WANG Wei, LUO Shi-Jie, XIAN Cong, XIAO Qun, YANG Yang, OU Yun, LIU Yun-Ya, XIE Shu-Hong. Enhanced Thermoelectric Properties of Hydrothermal Synthesized BiCl3/Bi2S3 Composites [J]. Journal of Inorganic Materials, 2019, 34(3): 328-334. |
| [6] | WANG Shu-Jiang, YANG Yong-Heng, WEN Chun-Yang, ZHANG Guo-Kui, YUAN Chun-Hui. Preparation and Property of Nano-Ag/illite Composite Material [J]. Journal of Inorganic Materials, 2018, 33(5): 570-576. |
| [7] | JIA Si-Qi, JIANG Zheng, CHI Li-Na, YE Ying, HU Shuang-Shuang. Synthesis and Photoelectrocatalytic Performance of Sb2S3 Nanorods from Natural Stibnite [J]. Journal of Inorganic Materials, 2018, 33(11): 1213-1218. |
| [8] | YANG Kun-Kun, YANG Shao-Hua, ZHAO Ping, ZHAO Yan-Long. Hydrothermal Synthesis of FeS2/Reduced Graphene Oxide Nanocomposite with Enhanced Discharge Performance for Thermal Battery [J]. Journal of Inorganic Materials, 2017, 32(7): 691-698. |
| [9] | GUO Yu, LI Dong-Xin, WU Hong-Mei, JIN Yu-Jia, ZHOU Li-Dai, CHEN Qiang-Qiang. Preparation, Characterization and Catalytic Performance of Supported Titanium Silicalite-1 Zeolite Membrane Catalyst [J]. Journal of Inorganic Materials, 2017, 32(6): 631-636. |
| [10] | ABUBAKER Abutartour, LOTFIA El-Majdoub, SHI Ya-Sai, LI Ni-Li, XU Qing-Hong. A New Porous Zirconium Phosphonate Hybride Material and Its Adsorption Properties [J]. Journal of Inorganic Materials, 2017, 32(3): 305-312. |
| [11] | LI Jin-Kai, TENG Xin, CAO Bing-Qiang, LIU Zong-Ming. Synthesis and Property of Spherical Lu3Al5O12:Eu3+ Phosphor [J]. Journal of Inorganic Materials, 2016, 31(6): 634-640. |
| [12] | LI Jun, PAN Lei, WANG Ji-Tong, LONG Dong-Hui, QIAO Wen-Ming, LING Li-Cheng. Low-temperature Removal of NO by Spherical Activated Carbon Loaded with MnOx-CeO2 and Melamine [J]. Journal of Inorganic Materials, 2016, 31(11): 1205-1211. |
| [13] | ZHANG Chuan, WANG Ji-Tong, LI Xu, LONG Dong-Hui, QIAO Wen-Ming, LING Li-Cheng. Facile Preparation, Structural Control and Spheroidization of Mesoporous Carbons Using Hydrolyzed Water Glass as a Template [J]. Journal of Inorganic Materials, 2015, 30(8): 848-854. |
| [14] | XIE Hui-Dong, LI Fei, CHEN Chao, XI Hai-Hong, SHI Ling. Microwave Dielectric Properties of LaPO4 Ceramics Synthesized by a Hydrothermal Method [J]. Journal of Inorganic Materials, 2015, 30(8): 882-886. |
| [15] | ZHEN Yan-Zhong, LI Jing, WANG Dan-Jun, FU Feng, XUE Gang-Lin. Synthesis of α-MoO3 Nanobelt and Its Photocatalytic Oxidative Desulfurization(Photo-ODS) Activity of Simulation Fuel [J]. Journal of Inorganic Materials, 2015, 30(4): 408-412. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||