
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (9): 924-930.DOI: 10.15541/jim20140021
• Orginal Article • Previous Articles Next Articles
XIANG Li1, 2, DING Xiao-Fang1, 2
Received:2014-01-07
Revised:2014-03-28
Published:2014-09-17
Online:2014-08-21
CLC Number:
XIANG Li, DING Xiao-Fang. Influence of NH4HCO3 on Microstructure of Ni/La10Si5.8Mg0.2O26.8 Anode[J]. Journal of Inorganic Materials, 2014, 29(9): 924-930.
| Sample | Start materials | Weight ratio |
|---|---|---|
| 0 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:0 |
| 1 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:1 |
| 2 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:2 |
| 3 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:3 |
| 4 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:4 |
Table 1 Weight ratio of start materials for anode samples
| Sample | Start materials | Weight ratio |
|---|---|---|
| 0 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:0 |
| 1 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:1 |
| 2 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:2 |
| 3 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:3 |
| 4 | La10Si5.8Mg0.2O26.8:NiO:NH4HCO3 | 4:6:4 |
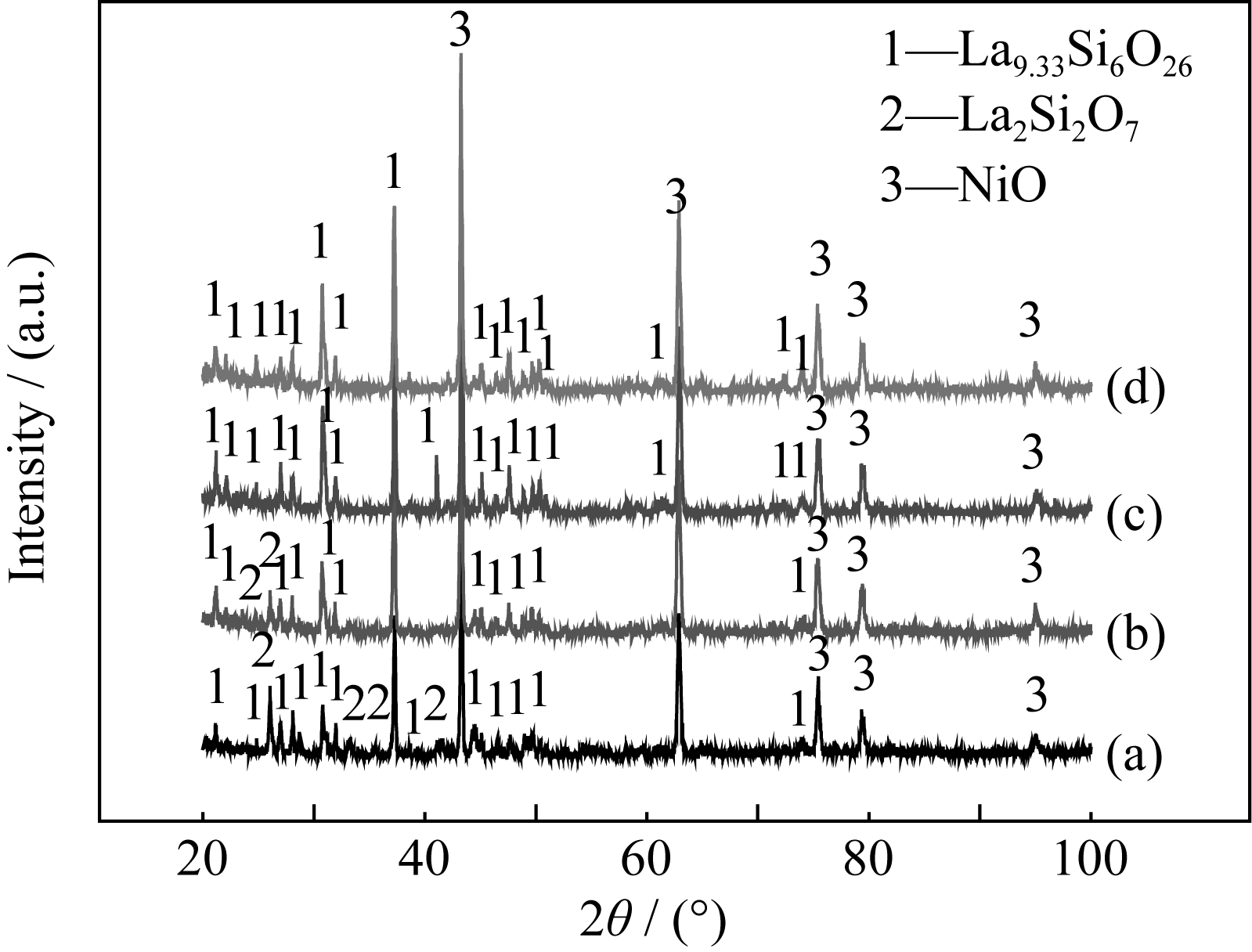
Fig. 5 XRD patterns of samples with different amounts of NH4HCO3 before reduction (a) 10wt% NH4HCO3; (b) 20wt% NH4HCO3; (c) 30wt% NH4HCO3; (d) 40wt% NH4HCO3
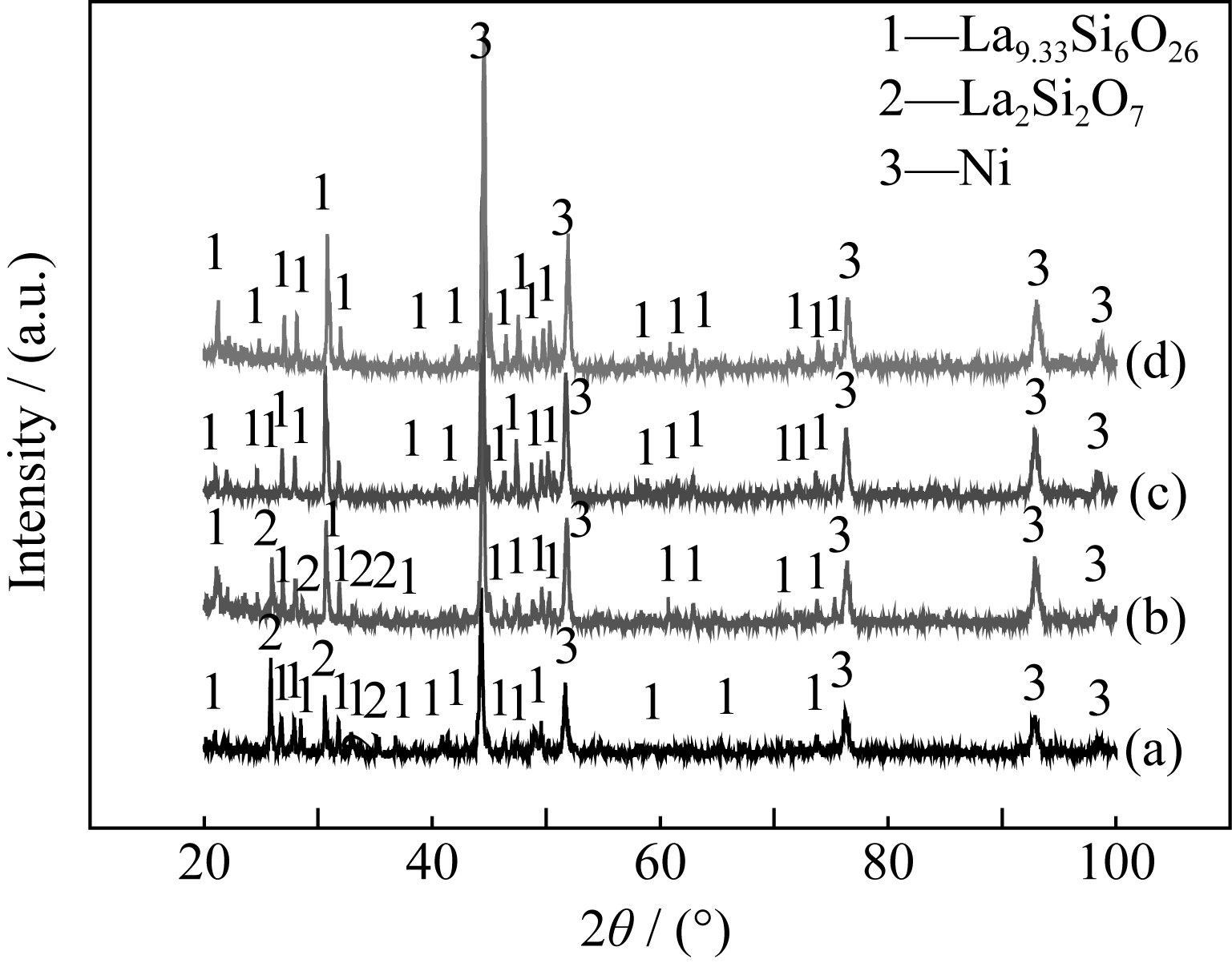
Fig. 6 XRD patterns of samples with different amounts of NH4HCO3 after reduction (a) 10wt% NH4HCO3; (b) 20wt% NH4HCO3; (c) 30wt% NH4HCO3; (d) 40wt% NH4HCO3
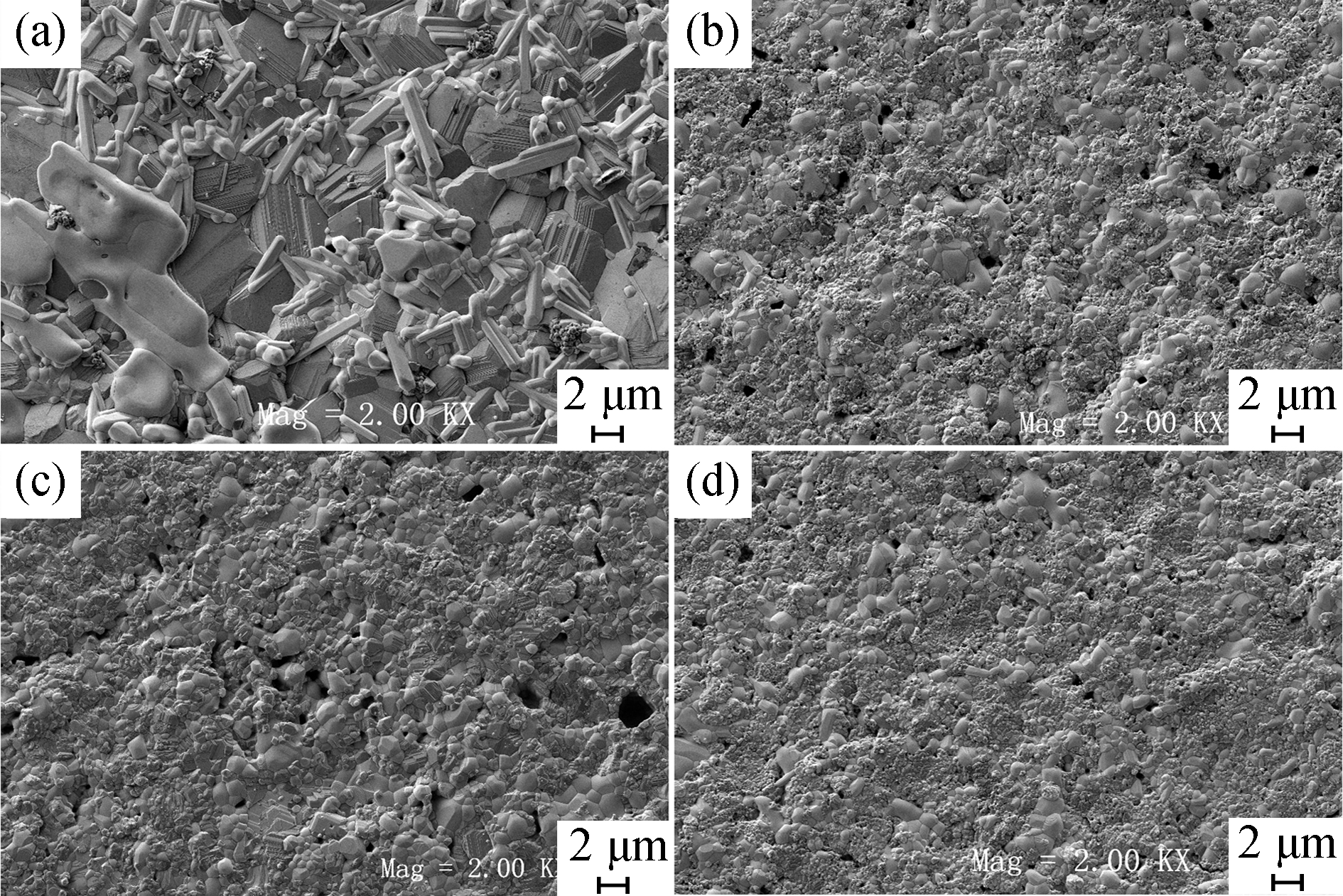
Fig. 7 FE-SEM surface micrographs of samples with different amount of NH4HCO3 before reduction (a) 10wt% NH4HCO3; (b) 20wt% NH4HCO3; (c) 30wt% NH4HCO3; (d) 40wt% NH4HCO3

Fig. 8 FE-SEM surface micrographs of samples with different amount of NH4HCO3 after reduction (a) 10wt% NH4HCO3; (b) 20wt% NH4HCO3; (c) 30wt% NH4HCO3; (d) 40wt% NH4HCO3
| Amount of NH4HCO3 | 10wt% | 20wt% | 30wt% | 40wt% |
|---|---|---|---|---|
| Resistance/Ω | 2.38 | 2.17 | 1.78 | 1.4×107 |
Table 2 Resistance of anodes with different amount of NH4HCO3
| Amount of NH4HCO3 | 10wt% | 20wt% | 30wt% | 40wt% |
|---|---|---|---|---|
| Resistance/Ω | 2.38 | 2.17 | 1.78 | 1.4×107 |
| [1] | NAKAYAMA S, KAGEYAMA T, AONO H, et al. Ionic conductivity of lanthanoid silicates, Ln10(SiO4)6O3 (Ln= La, Nd, Sm, Gd, Dy, Y, Ho, Er and Yb). Journal of Materials Chemistry, 1995, 5(11): 1801-1805. |
| [2] | NAKAYAMA S, SAKAMOTO M. Electrical properties of new type high oxide ionic conductor RE10Si6O27 (RE= La, Pr, Nd, Sm, Gd, Dy). Journal of the European Ceramic Society, 1998, 18: 1413-1418. |
| [3] | BÉCHADE E, MASSON O, IWATA T, et al. Diffusion path and conduction mechanism of oxide ions in apatite-type lanthanum silicates. Chemistry of Materials, 2009, 21: 2508-2517. |
| [4] | ALI R, YASHIMA M, MATSUSHITA Y, et al. Diffusion path of oxide ions in an apatite-type ionic conductor La9.69(Si5.70Mg0.30)O26.24. Chemistry of Materials, 2008, 20: 5203-5208. |
| [5] | LEÓN-REINA L, PORRAS-VÁZQUEZ J M, LOSILLA E R, et al. Low temperature crystal structures of apatite oxygen-conductors containing interstitial oxygen. Dalton Transactions, 2007, 2058-2064. |
| [6] | MATSUNAGA K, TOYOURA K. First-principles analysis of oxide- ion conduction mechanism in lanthanum silicate. Journal of Materials Chemistry, 2012, 22: 7265-7273. |
| [7] | KHARTON V V, MARQUES F M B, ATKINSON A. Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ionics, 2004, 174: 135-149. |
| [8] | Jacobson A J. Materials for Solid Oxide Fuel Cells. Chemistry of Materials, 2010, 22: 660-674. |
| [9] | SHAULA A L, KHARTON V V, MARQUES F M B. Ionic and electronic conductivities, stability and thermal expansion of La10-x(Si, Al)6O26±δ solid electrolytes. Solid State Ionics, 2006, 177: 1725-1728. |
| [10] | NAKAO T, MINESHIGE A, KOBUNE M, et al. Chemical stability of La10Si6O27 and its application to electrolytes for solid oxide fuel cells. Solid State Ionics, 2008, 179: 1567-1569. |
| [11] | KENDRICK E, ISLAM M S, SLATER P R. Developing apatites for solid oxide fuel cells: insight into structural, transport and doping properties. Journal of Materials Chemistry, 2007, 17: 3104-3111. |
| [12] | YOSHIOKA H, NOJIRI Y, TANASE S. Ionic conductivity and fuel cell properties of apatite-type lanthanum silicates doped with Mg and containing excess oxide ions. Solid State Ionics, 2008, 179: 2165-2169. |
| [13] | BRISSE A, SAUVET A L, BARTHET C, et al. Electrochemical characterizations of Ni/doped lanthanum silicates cermets deposited by spin coating. Fuel Cell, 2006, 6(1): 59-63. |
| [14] | SAVIGNAT S B, CHIRON M, BARTHET C. Tape casting of new eletrolyte and anode materials for SOFCs operated at intermediate temperature. Journal of the European Ceramic Society, 2007, 27: 673-678. |
| [15] | 牛江坡. 固体氧化物燃料电池复合阳极材料的制备和性能研究. 长春: 吉林大学硕士学位论文, 2008: 16-17. |
| [16] | JOTHINATHAN E, VANMEENSEL K, VLEUGELS J, et al. Apatite type lanthanum silicate and composite anode half cells. Solid State Ionics, 2011, 192: 419-423. |
| [17] | YAN J W, DONG Y L, YU C Y, et al. Fabrication and characterization of anode substrates and supported electrolyte thin films for intermediate temperature solid oxide fuel cells. Journal of Inorganic Materials, 2001, 16(5): 804-814. |
| [18] | CLEMMER R M C, CORBIN S F. The influence of pore and Ni morphology on the electrical conductivity of porous Ni/YSZ composite anodes for use in solid oxide fuel cell applications. Solid State Ionics, 2009, 180: 721-730. |
| [19] | CHEN C C, LIU M F, YANG L, et al. Anode-supported micro-tubular SOFCs fabricated by a phase-inversion and dip-coating process. International Journal of Hydrogen Energy, 2011, 36: 5604-5610. |
| [20] | HU J Y, LÜ Z, CHEN K F, et al. Effect of composite pore-former on the fabrication and performance of anode-supported membranes for SOFCs. Journal of Membrane Science, 2008, 318: 445-451. |
| [21] | 韩敏芳,彭苏萍箸. 固体氧化物燃料电池材料及制备. 北京: 科学出版社, 2004: 141-143. |
| [22] | JIANG S P, ZHANG L, HE H Q, et al. Synthesis and characterization of Lanthanum silicate apatite by gel-casting route as electrolytes for solid oxide fuel cells. Journal of Power Sources, 2009, 189: 972-981. |
| [23] | DONG Y H, ZHOU X L, HUA X Z. Effect of porosity on thermal performance of Mo-Cu composite. Hot Working Technology, 2008, 37(16): 1-6. |
| [24] | 上海师范大学、上海化工学院《无机化学》编写组. 无机化学 (上册). 上海: 上海人民出版社, 1974: 243-246. |
| [25] | TIAN C A, LIU J L, CAI J, et al. Synthesis of La9.33Si6O26 single- phase ultrafine powder by Sol-Gel self-combustion method. Journal of Inorganic Materials, 2008, 23(1): 77-81. |
| [1] | QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi. Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4 [J]. Journal of Inorganic Materials, 2025, 40(5): 489-496. |
| [2] | MA Wen, SHEN Zhe, LIU Qi, GAO Yuanming, BAI Yu, LI Rongxing. Preparation of Y2O3 Coating by Suspension Plasma Spraying and Its Resistance to Plasma Etching [J]. Journal of Inorganic Materials, 2024, 39(8): 929-936. |
| [3] | PAN Jianlong, MA Guanjun, SONG Lemei, HUAN Yu, WEI Tao. High Stability/Catalytic Activity Co-based Perovskite as SOFC Anode: In-situ Preparation by Fuel Reducing Method [J]. Journal of Inorganic Materials, 2024, 39(8): 911-919. |
| [4] | SHEN Bin, ZHANG Xu, XIONG Huai, LI Haiyuan, XIE Xinglong. Preparation and Optical Properties of Sol-Gel SiO2 Antireflective Films [J]. Journal of Inorganic Materials, 2024, 39(5): 525-530. |
| [5] | XIAO Wenyan, FU Yan, YANG Shubin, ZHU Jie, CHENG Zhaoyang, WEN Xiaoxu, TANG Jiafan, YU Liang, ZHANG Qian. Seawater Electrolysis Performance of Self-supported Amorphous Ce-FeHPi/NF Electrode [J]. Journal of Inorganic Materials, 2024, 39(12): 1348-1356. |
| [6] | DAI Le, LIU Yang, GAO Xuan, WANG Shuhao, SONG Yating, TANG Mingmeng, DMITRY V Karpinsky, LIU Lisha, WANG Yaojin. Self-polarization Achieved by Compositionally Gradient Doping in BiFeO3 Thin Films [J]. Journal of Inorganic Materials, 2024, 39(1): 99-106. |
| [7] | HU Mengfei, HUANG Liping, LI He, ZHANG Guojun, WU Houzheng. Research Progress on Hard Carbon Anode for Li/Na-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(1): 32-44. |
| [8] | SU Nan, QIU Jieshan, WANG Zhiyu. F-doped Carbon Coated Nano-Si Anode with High Capacity: Preparation by Gaseous Fluorination and Performance for Lithium Storage [J]. Journal of Inorganic Materials, 2023, 38(8): 947-953. |
| [9] | JIA Yuna, CAO Xu, JIAO Xiuling, CHEN Dairong. Preparation of Alumina Ceramic Continuous Fibers with Inorganic Acidic Aluminum Sol as Precursor [J]. Journal of Inorganic Materials, 2023, 38(11): 1257-1264. |
| [10] | SU Nana, HAN Jingru, GUO Yinhao, WANG Chenyu, SHI Wenhua, WU Liang, HU Zhiyi, LIU Jing, LI Yu, SU Baolian. ZIF-8-derived Three-dimensional Silicon-carbon Network Composite for High-performance Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(9): 1016-1022. |
| [11] | SU Dongliang, CUI Jin, ZHAI Pengbo, GUO Xiangxin. Mechanism Study on Garnet-type Li6.4La3Zr1.4Ta0.6O12 Regulating the Solid Electrolyte Interphases of Si/C Anodes [J]. Journal of Inorganic Materials, 2022, 37(7): 802-808. |
| [12] | XIAO Meixia, LI Miaomiao, SONG Erhong, SONG Haiyang, LI Zhao, BI Jiaying. Halogenated Ti3C2 MXene as High Capacity Electrode Material for Li-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(6): 660-668. |
| [13] | WANG Yutong, ZHANG Feifan, XU Naicai, WANG Chunxia, CUI Lishan, HUANG Guoyong. Research Progress of LiTi2(PO4)3 Anode for Aqueous Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(5): 481-492. |
| [14] | RUAN Jing, YANG Jinshan, YAN Jingyi, YOU Xiao, WANG Mengmeng, HU Jianbao, ZHANG Xiangyu, DING Yusheng, DONG Shaoming. Porous SiC Ceramic Matrix Composite Reinforced by SiC Nanowires with High Strength and Low Thermal Conductivity [J]. Journal of Inorganic Materials, 2022, 37(4): 459-466. |
| [15] | WANG Jing, XU Shoudong, LU Zhonghua, ZHAO Zhuangzhuang, CHEN Liang, ZHANG Ding, GUO Chunli. Hollow-structured CoSe2/C Anode Materials: Preparation and Sodium Storage Properties for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(12): 1344-1350. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||