
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (3): 309-314.DOI: 10.3724/SP.J.1077.2014.13301
• Orginal Article • Previous Articles Next Articles
WANG Xiao-Yuan1, SHIMADA Takahiro2, KITAMURA Takayuki2
Received:2013-06-07
Revised:2013-09-20
Published:2014-03-20
Online:2014-02-18
CLC Number:
WANG Xiao-Yuan, SHIMADA Takahiro, KITAMURA Takayuki. First-principles Calculation on Ferroelectricity and Its Coupling Behavior with Mechanical Deformation of Ultrathin PbTiO3 Nanotube[J]. Journal of Inorganic Materials, 2014, 29(3): 309-314.
Add to citation manager EndNote|Ris|BibTeX
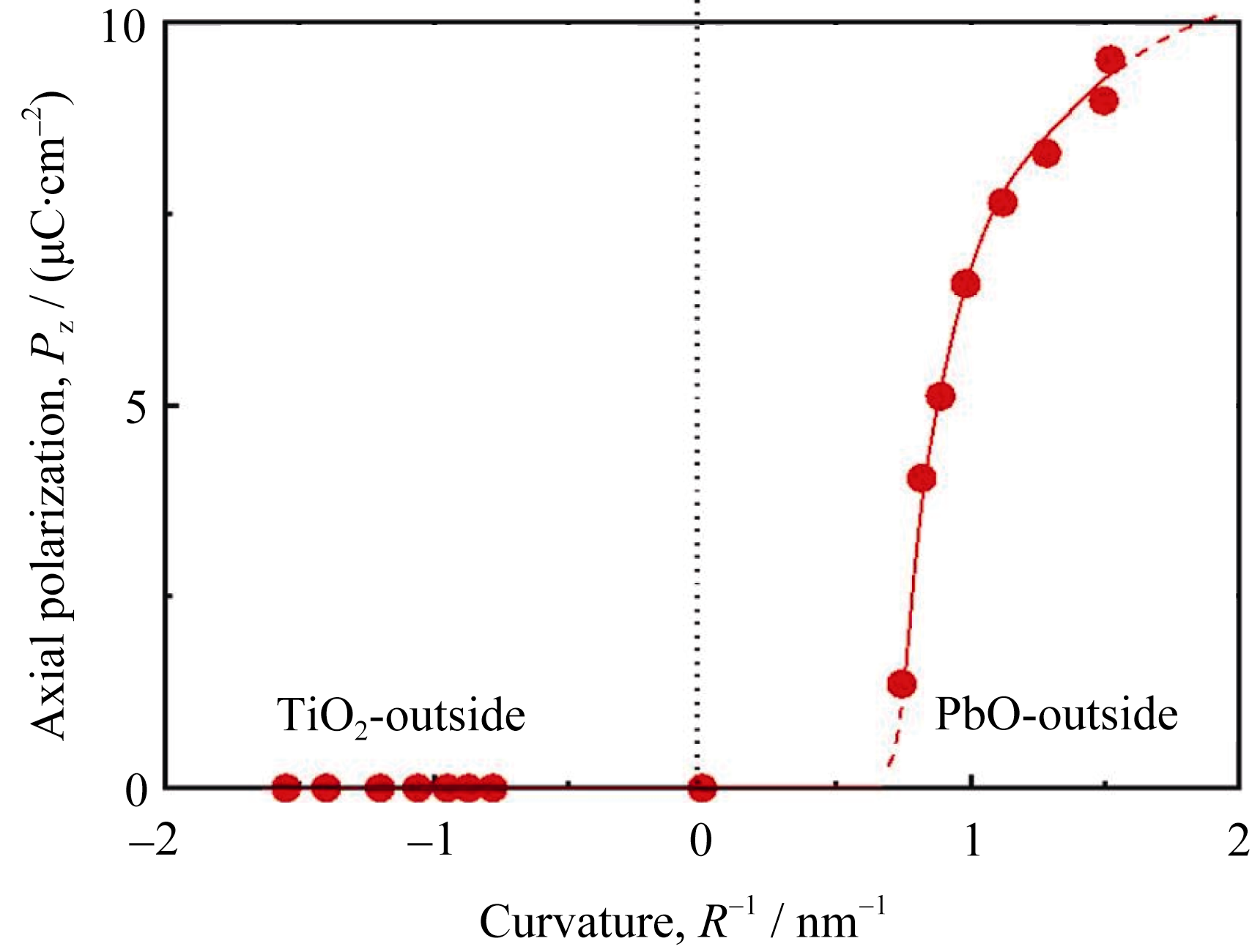
Fig. 3 Axial polarization (Pz,) as a function of nanotube curvature 1/R Positive and negative values represent the curvatures of the PbO-outside and TiO2-outside nanotubes, respectively
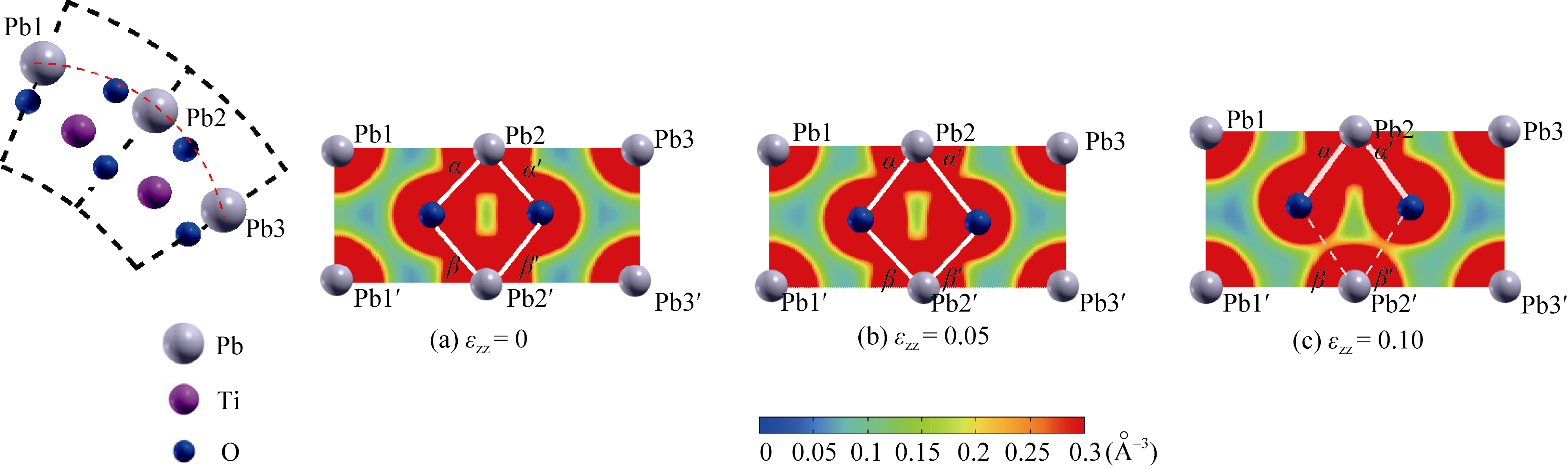
Fig. 5 Change in charge density distribution on the PbO layer of PbO-outside N=18 nanotube under axial tensile strain The covalent Pb-O bonds are emphasized by white lines
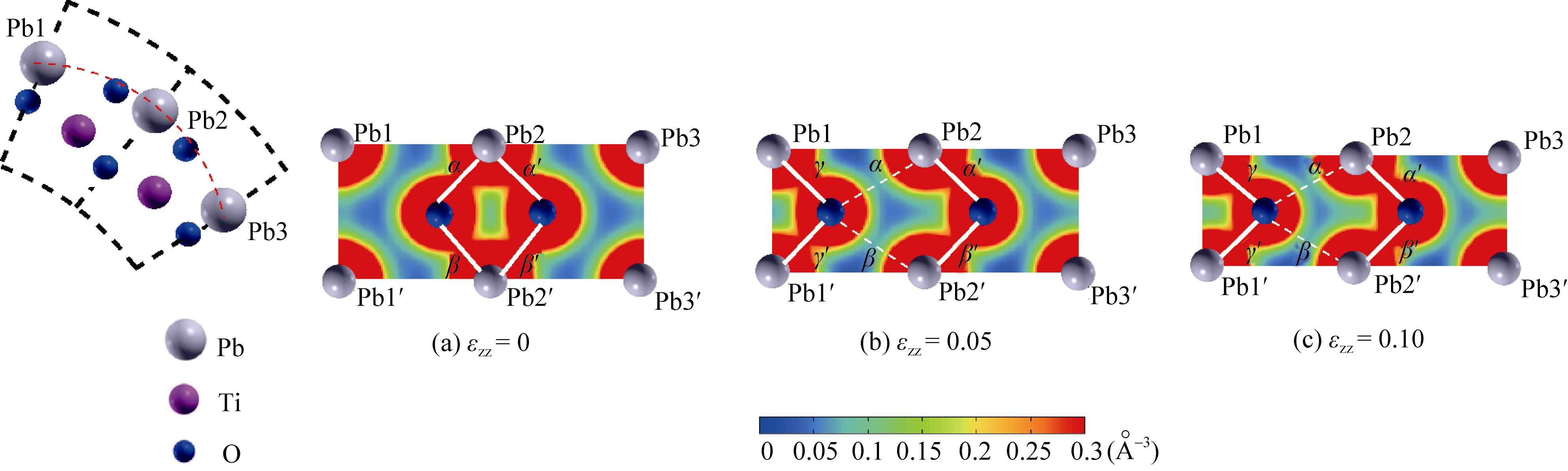
Fig. 6 Change in charge density distribution on the PbO layer of PbO-outside N=18 nanotube under axial compressive strain The covalent Pb-O bonds are emphasized by white lines
| Tension | Compression | |
|---|---|---|
| Critical stress/GPa | 7.09 | -8.06 |
| Fracture strain | 0.06 | -0.04 |
Table1 Critical stress and fracture strain of PbTiO3 nanotube in tension and compression
| Tension | Compression | |
|---|---|---|
| Critical stress/GPa | 7.09 | -8.06 |
| Fracture strain | 0.06 | -0.04 |
| [1] | 钟维烈. 铁电体物理学. 北京; 高等教育出版社, 1996: 7-105. |
| [2] | ZHU X, LIU Z, MING N. Perovskite oxide nanotubes: synthesis, structural characterization, properties and applications. J. Mater. Chem., 2010, 20: 4015-4030. |
| [3] | MAO Y, BANERJEE S, WONG S S. Hydrothermal synthesis of perovskite nanotubes. Chem. Commun., 2003, 3: 408-409. |
| [4] | RESTA R, POSTERNAK M, BALDERESCHI A. Towards a quantum theory of polarization in ferroelectrics: The case of KNbO3. Phys. Rev. Lett., 1993, 70: 1010-1013. |
| [5] | KRETSCHMER R, BINDER K. Surface effects on phase transitions in ferroelectrics and dipolar magnets. Phys. Rev. B, 1979, 20: 1065-1076. |
| [6] | ZHONG WEI-LIE, AI SHU-TAO, JIANG BIN. Two critical sizes of barium titanate and lead titanate. Journal of Inorganic Materials, 2002, 17(5): 1009-1012. |
| [7] | FONG D D, STEPHENSON G B, STREIFFER S K. Ferroelectricity in ultrathin perovskite films. Science, 2004, 304(5677): 1650-1653. |
| [8] | SAI N, KOLPAK A M, RAPPE A M. Ferroelectricity in ultrathin perovskite films. Phys. Rev. B, 2005, 72(2): 020101(R)-1-4. |
| [9] | UMENO Y, SHIMADA T, KITAMURA T, et al. Ab initio density functional theory study of strain effects on ferroelectricity at PbTiO3 surfaces. Phys. Rev. B, 2006, 74(17): 174111-1-9 |
| [10] | JUNQUERA J, GHOSEZ P. Critical thickness for ferroelectricity in perovskite ultrathin films. Nature, 2003, 422: 506-509. |
| [11] | BURCSU E, RAVICHANDRAN G, BHATTACHARYA K. Large strain electrostrictive actuation in barium titanate. Appl. Phys. Lett., 2000, 77(11): 1698-1700. |
| [12] | SHIMADA T, WANG X, TOMODA S, et al. Coexistence of rectilinear and vortex polarizations at twist boundaries in ferroelectric PbTiO3 from first principles. Phys. Rev. B, 2011, 83(9): 094121-1-9. |
| [13] | CEPERLEY D M, ALDER B J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett., 1980, 45(7): 566-569. |
| [14] | SHIMADA T, ISHII Y, KITAMURA T. Ab initio study of ferromagnetic single-wall nickel nanotubes. Phys. Rev. B, 2011, 84(16): 165452-1-6. |
| [15] | FERRARI A M, SZIBERTH D, NOEL Y. DFT modeling of anatase nanotubes. J. Mate. Chem., 2011, 21: 4568-4580. |
| [16] | SENGER R T, DAG S, CIRACI S. Chiral single-wall gold nanotubes. Phys. Rev. Lett., 2004, 93(19): 196807-1-4. |
| [17] | ZHONG W, KING-SMITH R D, VANDERBILT D. Giant LO-TO splittings in perovskite ferroeletrics. Phys. Rev. Lett., 1994, 72(22): 3618-3621. |
| [18] | MÜNCH I, HUBER E. A hexadomain vortex in tetragonal ferroelectrics. Appl. Phys. Lett., 2009, 95(2): 022913-1-3. |
| [19] | SCHILLING A, BYRNE D, GATALAN G, et al. Domains in ferroelectric nanodots. Nano Letters, 2009, 9(9): 3359-3364. |
| [20] | BOUSQUET E, DAWBER M, STUCKI N, et al. Improper ferroelectricity in perovskite oxide artificial superlattices. Nature, 2008, 452: 732-736. |
| [21] | COHEN R E. Origin of ferroelectricity in perovskite oxides. Nature,1992, 358: 136-138. |
| [22] | KUROIWA Y, AOYAGI S, SAWADA A, et al. Evidence for Pb-O covalency in tetragonal PbTiO3. Phys. Rev. Lett., 2001, 87(21): 217601-1-4 |
| [1] | ZHOU Yunkai, DIAO Yaqi, WANG Minglei, ZHANG Yanhui, WANG Limin. First-principles Calculation Study of the Oxidation Resistance of PANI Modified Ti3C2(OH)2 [J]. Journal of Inorganic Materials, 2024, 39(10): 1151-1158. |
| [2] | WU Xiaowei, ZHANG Han, ZENG Biao, MING Chen, SUN Yiyang. Comparison of Hybrid Functionals HSE and PBE0 in Calculating the Defect Properties of CsPbI3 [J]. Journal of Inorganic Materials, 2023, 38(9): 1110-1116. |
| [3] | WEN Zhiqin, HUANG Binrong, LU Taoyi, ZOU Zhengguang. Pressure on the Structure and Thermal Properties of PbTiO3: First-principle Study [J]. Journal of Inorganic Materials, 2022, 37(7): 787-794. |
| [4] | XIAO Meixia, LI Miaomiao, SONG Erhong, SONG Haiyang, LI Zhao, BI Jiaying. Halogenated Ti3C2 MXene as High Capacity Electrode Material for Li-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(6): 660-668. |
| [5] | LIU Xiao-Gen,BAO Yi-Wang,WAN De-Tian,SUN Yu-Kang. Critical Size on Spontaneous Breakage of Tempered Glass Initiated by NiS Particle [J]. Journal of Inorganic Materials, 2020, 35(2): 211-216. |
| [6] | LIN Qimin, CUI Jiangong, YAN Xin, YUAN Xueguang, CHEN Xiaoyu, LU Qichao, LUO Yanbin, HUANG Xue, ZHANG Xia, REN Xiaomin. First-principles Study on Electronic Structure and Optical Properties of Single Point Defect Graphene Oxide [J]. Journal of Inorganic Materials, 2020, 35(10): 1117-1122. |
| [7] | GU Feng, WANG You-Wei, ZHENG Zhi-Hui, LIU Jian-Jun, LU Wen-Cong. Catalytic Mechanism of Palladium Catalyst for the Oxidation Reduction and Evolution Reaction of Lithium-air Battery [J]. Journal of Inorganic Materials, 2018, 33(10): 1131-1135. |
| [8] | ZHANG Jian-Feng, CAO Hui-Yang, WANG Hong-Bing. Research Progress of Novel Two-dimensional Material MXene [J]. Journal of Inorganic Materials, 2017, 32(6): 561-570. |
| [9] | WANG Xiao-Yuan, YAN Ya-Bin, SHIMADA Takahiro, KITAMURA Takayuki. Research Progress in Atomistic Simulation on Ferroelectricity and Electromechanical Coupling Behavior of Nanoscale Ferroelectrics [J]. Journal of Inorganic Materials, 2015, 30(6): 561-570. |
| [10] | WANG Da-Wei, ZHAO Quan-Liang, CAO Mao-Sheng, CUI Yan, ZHANG Shu-Jun. Effect of Sn Content on the Phase Structure and Electrical Properties of PbSnO3-Pb(Mg1/3Nb2/3)O3-PbTiO3 Ternary Ceramics [J]. Journal of Inorganic Materials, 2014, 29(1): 28-32. |
| [11] | LI Hai-Min, GUO Hong-Li, LI Xue-Dong, LIU Guo, XIAO Ding-Quan, ZHU Jian-Guo. Effects of Different Annealing Technique on the Ferroelectric and Leakage Properties of 0.7BiFeO3-0.3PbTiO3 Thin Films [J]. Journal of Inorganic Materials, 2011, 26(10): 1053-1057. |
| [12] | SONG Hong-Zhang,LI Yong-Xiang,YIN Qing-Rui. Progress in Research on Critical Size of Phase Transition in Ferroelectrics [J]. Journal of Inorganic Materials, 2007, 22(4): 583-589. |
| [13] | XIN Jun,ZHENG Yan-Qing,SHI Er-Wei. Review and Prospect of First-principles Calculations on Piezoelectric Materials [J]. Journal of Inorganic Materials, 2007, 22(2): 193-200. |
| [14] | FENG Ji-Wei,ZHANG Wen-Qing,JIANG Wan. Atomistic Thermodynamic Simulation of Ag/Al2O3 Interfaces under O2 Pressure [J]. Journal of Inorganic Materials, 2007, 22(1): 119-122. |
| [15] | DONG Yan-Ling,DU Pi-Yi,WENG Wen-Jian,HAN Gao-Rong. In Situ Combination and Phase Formation of Sol-Gel Derived PbTiO3/(Ni,Pb) Ferrite Multiphase Powders [J]. Journal of Inorganic Materials, 2005, 20(5): 1071-1076. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||