
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (7): 823-829.DOI: 10.15541/jim20220688
Special Issue: 【能源环境】氢能材料(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
LI Guanglan( ), WANG Tianyu, LIU Yichen, LU Zhongfa
), WANG Tianyu, LIU Yichen, LU Zhongfa
Received:2022-11-17
Revised:2023-01-19
Published:2023-02-01
Online:2023-02-07
About author:LI Guanglan (1985-), female, PhD, associate professor. E-mail: guanglanli@dlut.edu.cn
Supported by:CLC Number:
LI Guanglan, WANG Tianyu, LIU Yichen, LU Zhongfa. Layered NiFeCo-LDH-Ti6C3.75 Catalyst: Preparation and Performance for Oxygen Evolution Reaction[J]. Journal of Inorganic Materials, 2023, 38(7): 823-829.
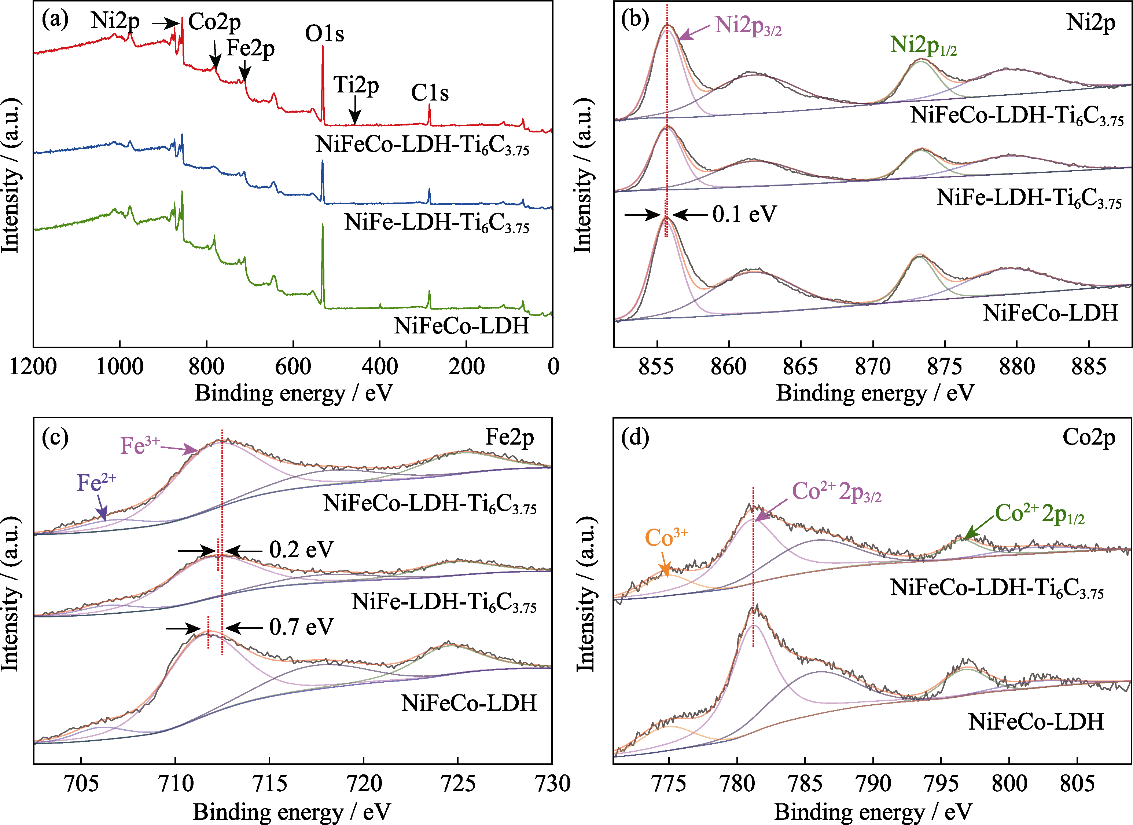
Fig. 5 (a) Total survey, high resolution (b) Ni2p and (c) Fe2p XPS spectra for NiFeCo-LDH-Ti6C3.75, NiFe LDH-Ti6C3.75 and NiFeCo-LDH catalysts, (d) Co2p XPS spectra for NiFeCo-LDH-Ti6C3.75 and NiFeCo-LDH catalysts
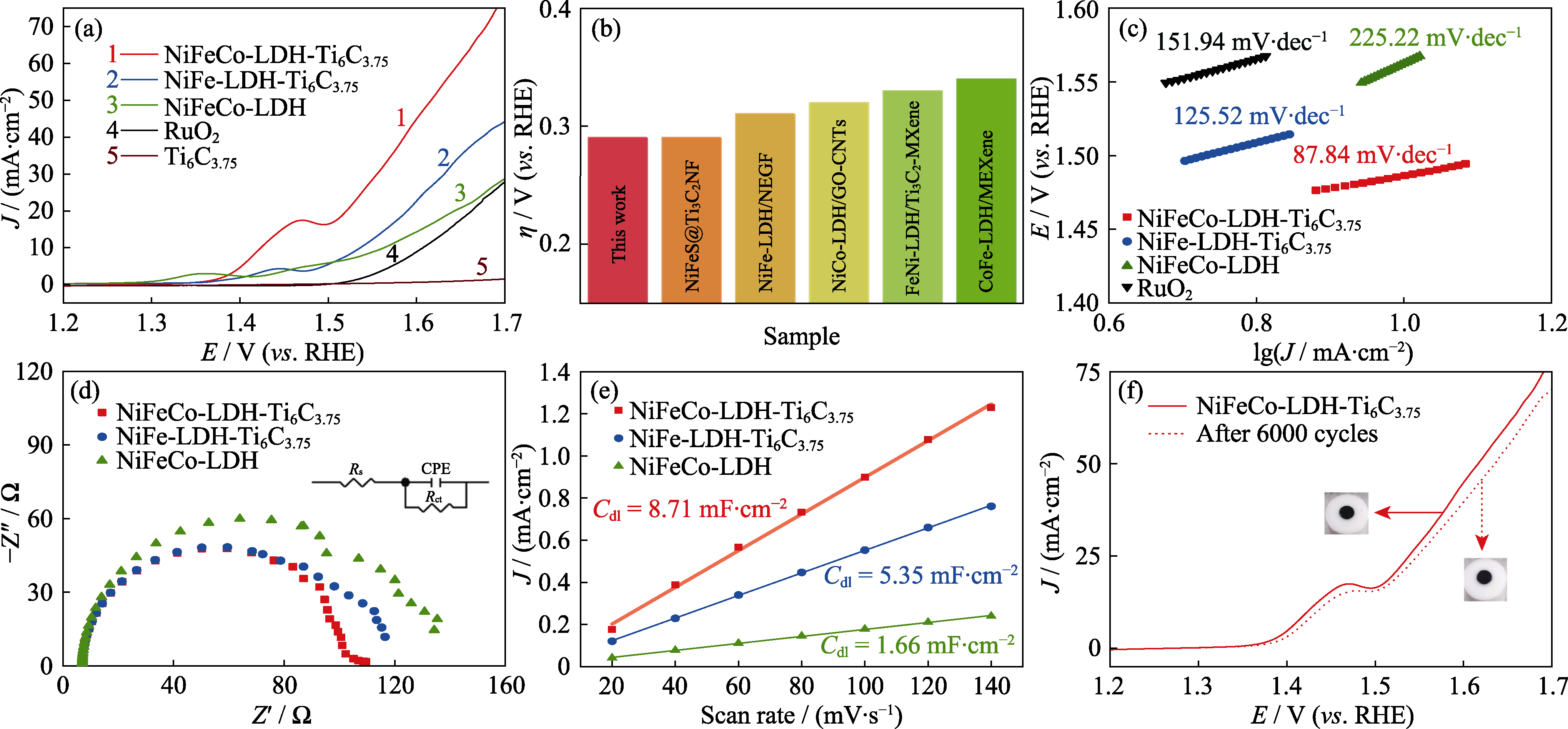
Fig. 6 Electrochemical performance of NiFeCo-LDH-Ti6C3.75, NiFe-LDH-Ti6C3.75, NiFeCo-LDH, and RuO2 catalysts (a) LSV curves, (b) overpotential at 20and 50 mA·cm-2, and (c) Tafel slopes of NiFeCo-DH-Ti6C3.75, NiFe LDH-Ti6C3.75, NiFeCo-LDH, and RuO2 catalysts; (d) EIS and (e) Cdl of NiFeCo-DH-Ti6C3.75, NiFe LDH-Ti6C3.75, and NiFeCo-LDH; (f) Accelerated aging curves of NiFeCo-LDH-Ti6C3.75 before and after 6000 cycles
| [1] |
LUO Y T, ZHANG Z Y, CHHOWALLA M, et al. Recent advances in design of electrocatalysts for high-current-density water splitting. Advanced Materials, 2022, 34(16):2108133.
DOI URL |
| [2] |
LI Y, WANG H H, PRIEST C, et al. Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions. Advanced Materials, 2021, 33(6):2000381.
DOI URL |
| [3] |
NWABARA U O, COFELL E R, VERMA S, et al. Durable cathodes and electrolyzers for the efficient aqueous electrochemical reduction of CO2. ChemSusChem, 2020, 13(5):855.
DOI PMID |
| [4] |
FANG Y X, HOU Y D, FU X Z, et al. Semiconducting polymers for oxygen evolution reaction under light illumination. Chemical Reviews, 2022, 122(3):4204.
DOI PMID |
| [5] |
ZHANG L J, JANG H S, LIU H H, et al. Sodium-decorated amorphous/crystalline RuO2 with rich oxygen vacancies: a robust pH-universal oxygen evolution electrocatalyst. Angewandte Chemie- International Edition, 2021, 60(34):18821.
DOI URL |
| [6] |
OVER H. Fundamental studies of planar single-crystalline oxide model electrodes(RuO2, IrO2) for acidic water splitting. ACS Catalysis, 2021, 11(14):8848.
DOI URL |
| [7] | GONG T Y, ZHANG J Y, LIU Y, et al. Construction of hetero- phase Mo2C-CoO@N-CNFs film as a self-supported Bi-functional catalyst towards overall water splitting. Chemical Engineering Journal, 2022, 451: 139025. |
| [8] | HE J, ZHOU X, XU P, et al. Promoting electrocatalytic water oxidation through tungsten-modulated oxygen vacancies on hierarchical FeNi-layered double hydroxide. Nano Energy, 2021, 80: 105540. |
| [9] |
ZHENG F Q, ZHANG W F, ZHANG X X, et al. Sub-2 nm ultrathin and robust 2D FeNi layered double hydroxide nanosheets packed with 1D FeNi-MOFs for enhanced oxygen evolution electrocatalysis. Advanced Functional Materials, 2021, 31(43):2103318.
DOI URL |
| [10] | QIAO C, USMAN Z, CAO T, et al. High-valence Ni and Fe sites on sulfated NiFe-LDH nanosheets to enhance O-O coupling for water oxidation. Chemical Engineering Journal, 2021, 426: 130873. |
| [11] |
LIN Y P, WANG H, PENG C K, et al. Co-induced electronic optimization of hierarchical NiFe LDH for oxygen evolution. Small, 2020, 16(38):2002426.
DOI URL |
| [12] | WANG Y Q, TAO S, LIN H, et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy, 2021, 81: 105606. |
| [13] | BABAR P, LOKHANDE A, KARADE V, et al. Bifunctional 2D electrocatalysts of transition metal hydroxide nanosheet arrays for water splitting and urea electrolysis. ACS Sustainable Chemistry & Engineering, 2019, 7(11):10035. |
| [14] | YANG Y, WEI S Y, LI Y F, et al. Effect of cobalt doping-regulated crystallinity in nickel-iron layered double hydroxide catalyzing oxygen evolution. Applied Catalysis B: Environmental, 2022, 314: 121491. |
| [15] |
ZHOU D J, CAI Z, JIA Y, et al. Activating basal plane in NiFe layered double hydroxide by Mn2+ doping for efficient and durable oxygen evolution reaction. Nanoscale Horizons, 2018, 3(5):532.
DOI URL |
| [16] |
LEE J, JUNG H, PARK Y, et al. High-efficiency anion-exchange membrane water electrolyzer enabled by ternary layered double hydroxide anode. Small, 2021, 17(28):2100639.
DOI URL |
| [17] | ZHOU Y, ZHANG W B, HU J L, et al. Inherent oxygen vacancies boost surface reconstruction of ultrathin Ni-Fe layered-double- hydroxides toward efficient electrocatalytic oxygen evolution. ACS Sustainable Chemistry & Engineering, 2021, 9(21):7390. |
| [18] | YE Y, GAN Y H, CAI R, et al. Oxygen vacancies and surface reconstruction on NiFe LDH@Ni(OH)2 heterojunction synergistically triggering oxygen evolution and urea oxidation reaction. Journal of Alloys and Compounds, 2022, 921: 166145. |
| [19] | YANG R, ZHOU Y M, XING Y Y, et al. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Applied Catalysis B: Environmental, 2019, 253: 131. |
| [20] | SU H, JIANG J, LI N, et al. NiCu alloys anchored defect-rich NiFe layered double-hydroxides as efficient electrocatalysts for overall water splitting. Chemical Engineering Journal, 2022, 446: 137226. |
| [21] | WEN Y Y, WEI Z T, LIU J H, et al. Synergistic cerium doping and MXene coupling in layered double hydroxides as efficient electrocatalysts for oxygen evolution. Journal of Energy Chemistry, 2021, 52: 412. |
| [22] | YU M Z, ZHENG J Q, GUO M. La-doped NiFe-LDH coupled with hierarchical vertically aligned MXene frameworks for efficient overall water splitting. Journal of Energy Chemistry, 2022, 70: 472. |
| [23] | SHEN J, ZHANG P, XIE R S, et al. Controlled self-assembled NiFe layered double hydroxides/reduced graphene oxide nanohybrids based on the solid-phase exfoliation strategy as an excellent electrocatalyst for the oxygen evolution reaction. ACS Applied Materials & Interfaces, 2019, 11(14):13545. |
| [24] | YIN X, HUA Y N, HAO W B, et al. Hierarchical nanocomposites of nickel/iron-layered double hydroxide ultrathin nanosheets strong-coupled with nanocarbon networks for enhanced oxygen evolution reaction. Electrochimica Acta, 2022, 420: 140455. |
| [25] | AMBRIZ-PELAEZ O, BEJAR J, RAMOS-CASTILLO M, et al. Defected NiFe layered double hydroxides on N-doped carbon nanotubes as efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Applied Surface Science, 2022, 601: 154253. |
| [26] | LI G L, CAO S, LU Z F, et al. FePc nanoclusters modified NiCo layered double hydroxides in parallel with Ti3C2 MXene as a highly efficient and durable bifunctional oxygen electrocatalyst for zinc-air batteries. Applied Surface Science, 2022, 591: 153142. |
| [27] | LIAO F F, YANG G Y, CHENG Q H, et al. Rational design and facile synthesis of Ni-Co-Fe ternary LDH porous sheets for high-performance aqueous asymmetric supercapacitor. Electrochimica Acta, 2022, 428: 140939. |
| [28] | HE H L, LÜ S W, KANG Y, et al. In situ preparation of NiCoFe- LDH nanoflowers on carbon cloth toward simultaneous detecting hydroquinone and catechol. Journal of Electroanalytical Chemistry, 2022, 919: 116540. |
| [29] | ZHAO P P, NIE H Q, ZHOU Z R, et al. NiFe-LDH grown on three-dimensional Cu3P nano-array for highly efficient water oxidation. ChemistrySelect, 2018, 3: 8064. |
| [30] | ROY A, TARIQ M Z, LA M, et al. A comparative study on the oxygen evolution reaction of cobalt and nickel based hydroxide electrodes in alkaline electrolyte. Journal of Electroanalytical Chemistry, 2022, 920: 116633. |
| [31] | CHEN J, REN Y J, ZHANG H Y, et al. Ni-Co-Fe layered double hydroxide coated on Ti3C2MXene for high-performance asymmetric supercapacitor. Applied Surface Science, 2021, 562: 150116. |
| [32] | LI X T, LIU Y Z, SUN Q D, et al. Effect of cationic and anionic defects on NiFe LDH in electrocatalytic oxygen evolution. ACS Sustainable Chemistry & Engineering, 2022, 10(44):14474. |
| [33] | CHANDA D, KANNAN K, GAUTAM J, et al. Effect of the interfacial electronic coupling of nickel-iron sulfide nanosheets with layer Ti3C2 MXenes as efficient bifunctional electrocatalysts for anion-exchange membrane water electrolysis. Applied Catalysis B: Environmental, 2023, 321: 122039. |
| [34] | MANNA N, AYASHA N, SINGH S K, et al. A NiFe layered double hydroxide-decorated N-doped entangled-graphene framework: a robust water oxidation electrocatalyst. Nanoscale Advances, 2020, 2(4):1709. |
| [35] |
YIN P Q, WU G, WANG X Q, et al. NiCo-LDH nanosheets strongly coupled with GO-CNTs as a hybrid electrocatalyst for oxygen evolution reaction. Nano Research, 2021, 14(12):4783.
DOI |
| [36] | YU M Z, ZHOU S, WANG Z Y, et al. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy, 2018, 44: 181. |
| [37] | HAO C Y, WU Y, AN Y J, et al. Interface-coupling of CoFe-LDH on MXene as high-performance oxygen evolution catalyst. Material Today Energy, 2019, 12: 453. |
| [38] | YU Z Y, DUAN Y, FENG X Y, et al. Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Advanced Materials, 2021, 33: 2007100. |
| [1] | ZHANG Li-Juan, DI Lan-Bo, LI Yan-Chun, ZHANG Xiu-Ling. Preparation and Properties of Co-doped TiO2 with Assistance of Ionic Liquid [J]. Journal of Inorganic Materials, 2014, 29(8): 801-806. |
| [2] |
LI Yun-Jiao, XU Hu, KONG Long, LI Hua-Cheng, LI Chun-Xia, ZHANG Xian-Zhen, HAN Qiang.
Synthesis and Electrochemical Characterizations of Co-doped Lithium Manganese Oxide Spinel Li1.035Mn1.965O4 [J]. Journal of Inorganic Materials, 2014, 29(6): 661-666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||