
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (11): 1300-1308.DOI: 10.15541/jim20240532
LIU Panpan1( ), YAO Peng1, LIU Xuzi1, QU Li2, ZENG Lu1, SONG Zhaohua1, JIAO Yi1(
), YAO Peng1, LIU Xuzi1, QU Li2, ZENG Lu1, SONG Zhaohua1, JIAO Yi1( ), WANG Jianli1,2, CHEN Yaoqiang1,2
), WANG Jianli1,2, CHEN Yaoqiang1,2
Received:2024-12-23
Revised:2025-04-19
Published:2025-11-20
Online:2025-05-09
Contact:
JIAO Yi, associate professor. E-mail: jiaoyiscu@163.comAbout author:LIU Panpan (2000-), male, Master candidate. E-mail: liupanpan@stu.scu.edu.cn
Supported by:CLC Number:
LIU Panpan, YAO Peng, LIU Xuzi, QU Li, ZENG Lu, SONG Zhaohua, JIAO Yi, WANG Jianli, CHEN Yaoqiang. MnOx/CeO2-ZrO2 Composite Oxides: Construction and Application in Soot Oxidation[J]. Journal of Inorganic Materials, 2025, 40(11): 1300-1308.
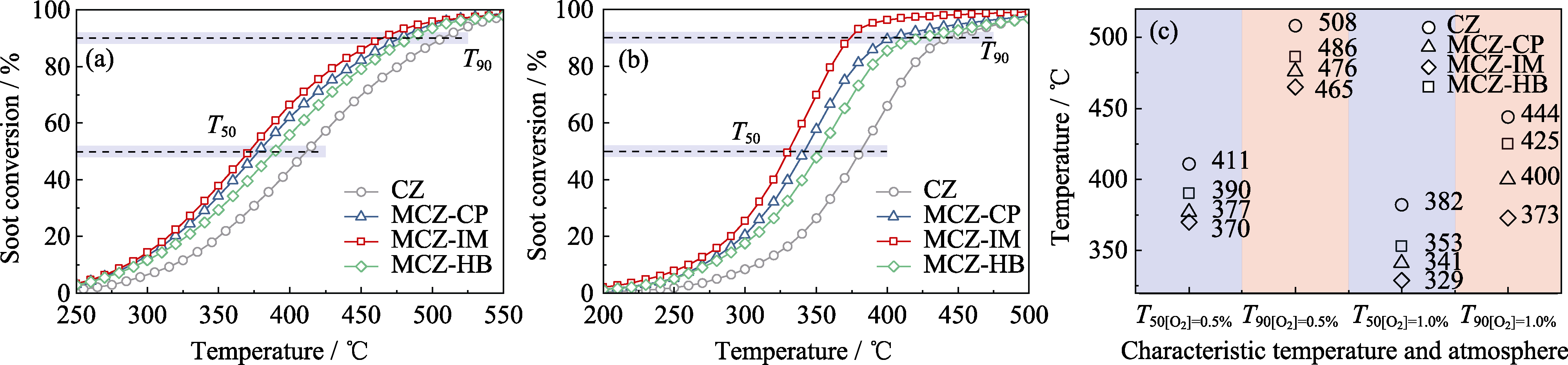
Fig. 1 Soot conversion, T50, and T90 of the catalysts under different oxygen concentrations with reaction conditions of flow rate at 500 mL/min and N2 balance (a, b) Soot conversion under (a) 0.5% and (b) 1.0% O2; (c) T50 and T90 under different oxygen concentrations
| Sample | Surface areaa /(m2•g-1) | Pore volumea /(mL•g-1) | Mean pore diametera /nm | Crystallinityb /% | Grain sizec /nm |
|---|---|---|---|---|---|
| CZ | 110.3 | 0.26 | 9.40 | 52.1 | 7.2 |
| MCZ-CP | 145.9 | 0.34 | 9.64 | 40.2 | 5.1 |
| MCZ-IM | 79.2 | 0.22 | 11.30 | 45.6 | 7.4 |
| MCZ-HB | 81.3 | 0.22 | 10.80 | 44.8 | 7.6 |
Table 1 Textural and structural properties of the prepared catalysts
| Sample | Surface areaa /(m2•g-1) | Pore volumea /(mL•g-1) | Mean pore diametera /nm | Crystallinityb /% | Grain sizec /nm |
|---|---|---|---|---|---|
| CZ | 110.3 | 0.26 | 9.40 | 52.1 | 7.2 |
| MCZ-CP | 145.9 | 0.34 | 9.64 | 40.2 | 5.1 |
| MCZ-IM | 79.2 | 0.22 | 11.30 | 45.6 | 7.4 |
| MCZ-HB | 81.3 | 0.22 | 10.80 | 44.8 | 7.6 |
| Sample | Mass fraction/% | |||
|---|---|---|---|---|
| O | Mn | Zr | Ce | |
| MCZ-CP | 24.38 | 2.98 | 22.44 | 50.20 |
| MCZ-IM | 24.13 | 7.54 | 23.14 | 45.18 |
| MCZ-HB | 21.52 | 1.03 | 24.58 | 52.87 |
Table 2 Surface elements composition of the catalysts
| Sample | Mass fraction/% | |||
|---|---|---|---|---|
| O | Mn | Zr | Ce | |
| MCZ-CP | 24.38 | 2.98 | 22.44 | 50.20 |
| MCZ-IM | 24.13 | 7.54 | 23.14 | 45.18 |
| MCZ-HB | 21.52 | 1.03 | 24.58 | 52.87 |

Fig. 6 Surface oxygen vacancies and active oxygen species of the samples (a) Raman spectra; (b-d) Ce3d (b), Mn2p (c), and O1s (d) XPS spectra; (e) DRIFTS spectra of O2 adsorption collected at 300 ℃; (f) Low-temperature EPR. Colorful figures are available on website
| Catalyst | Ce3+/Ce | Ce4+/Ce | Mn4+/Mn | (O22-+O2-)/OT | O2-/OT |
|---|---|---|---|---|---|
| CZ | 0.21 | 0.79 | — | 0.28 | 0.09 |
| MCZ-CP | 0.14 | 0.86 | 0.36 | 0.35 | 0.11 |
| MCZ-IM | 0.15 | 0.85 | 0.39 | 0.34 | 0.17 |
| MCZ-HB | 0.17 | 0.83 | 0.32 | 0.31 | 0.06 |
Table 3 Surface compositions and charge states of Ce, Mn and O species derived from XPS analysis
| Catalyst | Ce3+/Ce | Ce4+/Ce | Mn4+/Mn | (O22-+O2-)/OT | O2-/OT |
|---|---|---|---|---|---|
| CZ | 0.21 | 0.79 | — | 0.28 | 0.09 |
| MCZ-CP | 0.14 | 0.86 | 0.36 | 0.35 | 0.11 |
| MCZ-IM | 0.15 | 0.85 | 0.39 | 0.34 | 0.17 |
| MCZ-HB | 0.17 | 0.83 | 0.32 | 0.31 | 0.06 |
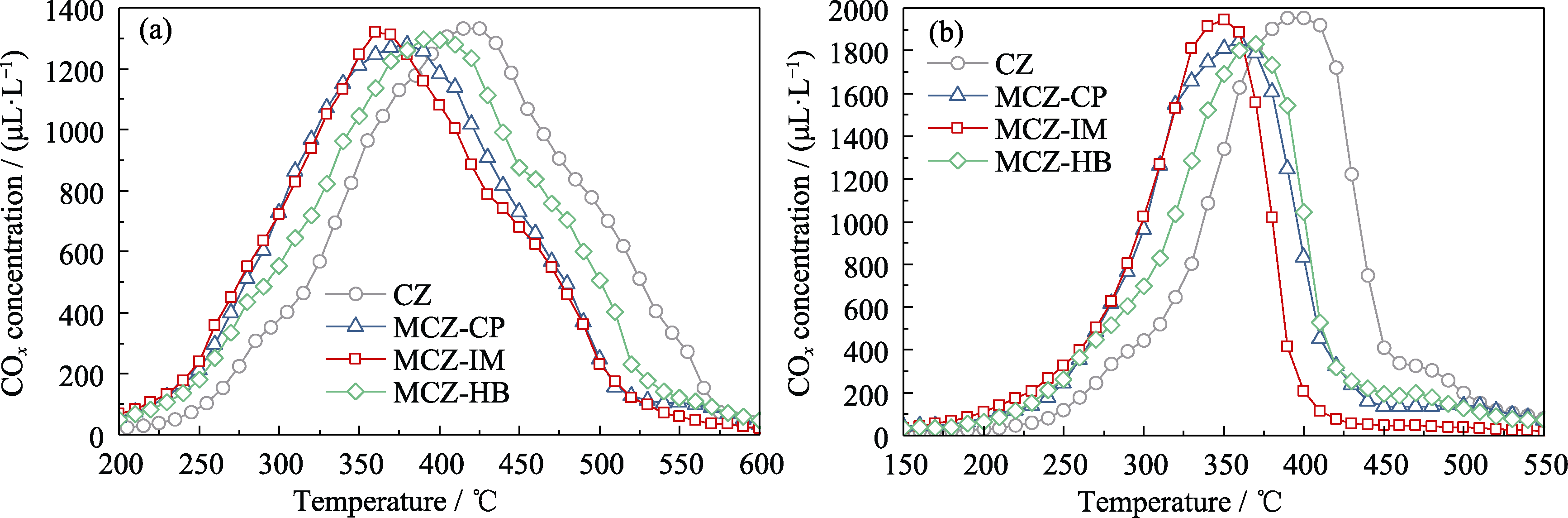
Fig. S1 Generated COx concentration of soot oxidation over the catalysts Reaction conditions: flow rate at 500 mL/min and N2 balance; [O2]: (a) 0.5% and (b) 1.0%
| Catalyst | Method | Reaction condition (gas and flow rate) | Heating rate/ (℃•min-1) | Catalyst and soot mass ratio | Contact mode | T50/Tmax/ ℃ | Ref. |
|---|---|---|---|---|---|---|---|
| MCZ-IM | Incipient wetness impregnation | 1% O2 | 5 | 10 : 1 | Tight | 329 | This work |
| 0.5% O2 (500 mL/min) | 370 | ||||||
| M10-CZ | Co-precipitation | 0.5% O2 (500 mL/min) | 5 | 10 : 1 | Loose | 520 | [S1] |
| Mn2O3 | Flame spray pyrolysis | 1% O2 + 2% H2O (500 mL/min) | 3.3 | 15 : 1 | Tight | 321 | [S4] |
| 10LM-CZ | Co-precipitation and citric acid complexation impregnation | 1% O2 (100 mL/min) | 5 | 10 : 1 | Tight | 362 | [S5] |
| 0.57Mn-CeO2 | Nitrate aerosol pyrolysis | 10% O2 (100 mL/min) | 10 | 4 : 1 | Tight | 355 | [S6] |
| 5 Mn-CP | Solution combustion synthesis | Air (100 mL/min) | 10 | 10 : 1 | Tight | 365 | [S7] |
| CM5 | EDTA-Citrate | Air (100 mL/min) | 10 | 10 : 1 | Tight | 360 | [S8] |
| Ce0.5Mn0.5O2 | Sol-gel | 12% O2 (100 mL/min) | 15 | 4 : 1 | Tight | 383 | [S9] |
| CMO_st | Solvothermal | - | 10 | 19 : 1 | Tight | 442 | [S10] |
| CM | Co-precipitation | Air (100 mL/min) | 10 | 4 : 1 | Tight | 396 | [S11] |
| CMC | Co-precipitation | Air (100 mL/min) | - | 4 : 1 | Tight | 363 | [S12] |
| Ce0.9Mn0.1 | Solid-phase grinding | 10% O2 (50 mL/min) | 10 | 10 : 1 | Tight | 389 | [S13] |
| Mn-Fib Ce | Plasma-assisted deposition | 18% O2 + 0.1%NO (20 mL/min) | 5 | 20 : 1 | Tight | 384 | [S14] |
Table S1 Comparison of catalytic activity with other studies
| Catalyst | Method | Reaction condition (gas and flow rate) | Heating rate/ (℃•min-1) | Catalyst and soot mass ratio | Contact mode | T50/Tmax/ ℃ | Ref. |
|---|---|---|---|---|---|---|---|
| MCZ-IM | Incipient wetness impregnation | 1% O2 | 5 | 10 : 1 | Tight | 329 | This work |
| 0.5% O2 (500 mL/min) | 370 | ||||||
| M10-CZ | Co-precipitation | 0.5% O2 (500 mL/min) | 5 | 10 : 1 | Loose | 520 | [S1] |
| Mn2O3 | Flame spray pyrolysis | 1% O2 + 2% H2O (500 mL/min) | 3.3 | 15 : 1 | Tight | 321 | [S4] |
| 10LM-CZ | Co-precipitation and citric acid complexation impregnation | 1% O2 (100 mL/min) | 5 | 10 : 1 | Tight | 362 | [S5] |
| 0.57Mn-CeO2 | Nitrate aerosol pyrolysis | 10% O2 (100 mL/min) | 10 | 4 : 1 | Tight | 355 | [S6] |
| 5 Mn-CP | Solution combustion synthesis | Air (100 mL/min) | 10 | 10 : 1 | Tight | 365 | [S7] |
| CM5 | EDTA-Citrate | Air (100 mL/min) | 10 | 10 : 1 | Tight | 360 | [S8] |
| Ce0.5Mn0.5O2 | Sol-gel | 12% O2 (100 mL/min) | 15 | 4 : 1 | Tight | 383 | [S9] |
| CMO_st | Solvothermal | - | 10 | 19 : 1 | Tight | 442 | [S10] |
| CM | Co-precipitation | Air (100 mL/min) | 10 | 4 : 1 | Tight | 396 | [S11] |
| CMC | Co-precipitation | Air (100 mL/min) | - | 4 : 1 | Tight | 363 | [S12] |
| Ce0.9Mn0.1 | Solid-phase grinding | 10% O2 (50 mL/min) | 10 | 10 : 1 | Tight | 389 | [S13] |
| Mn-Fib Ce | Plasma-assisted deposition | 18% O2 + 0.1%NO (20 mL/min) | 5 | 20 : 1 | Tight | 384 | [S14] |
| Sample | Element | Atomic fraction/% | Mass fraction/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Average | Area 1 | Area 2 | Area 3 | Average | ||
| MCZ-CP | O | 62.48 | 66.22 | 69.82 | 66.17 | 21.55 | 24.14 | 27.45 | 24.38 |
| Mn | 2.49 | 2.85 | 2.92 | 2.75 | 2.52 | 3.06 | 3.37 | 2.98 | |
| Zr | 15.70 | 11.73 | 10.37 | 12.60 | 26.47 | 20.91 | 19.94 | 22.44 | |
| Ce | 19.33 | 19.20 | 16.89 | 18.47 | 49.45 | 51.90 | 49.24 | 50.20 | |
| MCZ-IM | O | 60.16 | 63.95 | 68.43 | 64.18 | 21.21 | 24.43 | 26.76 | 24.13 |
| Mn | 6.91 | 8.73 | 4.92 | 6.86 | 7.16 | 9.81 | 5.66 | 7.54 | |
| Zr | 16.25 | 12.07 | 9.86 | 12.73 | 28.02 | 22.54 | 18.86 | 23.14 | |
| Ce | 16.68 | 15.25 | 16.80 | 16.24 | 43.61 | 43.22 | 48.72 | 45.18 | |
| MCZ-HB | O | 61.05 | 65.04 | 63.64 | 63.24 | 20.63 | 22.36 | 21.58 | 21.52 |
| Mn | 0.89 | 0.91 | 1.30 | 1.03 | 0.88 | 0.92 | 1.30 | 1.03 | |
| Zr | 19.84 | 11.62 | 12.91 | 14.79 | 32.79 | 19.54 | 21.41 | 24.58 | |
| Ce | 18.23 | 22.43 | 22.15 | 20.94 | 45.70 | 57.19 | 55.71 | 52.87 | |
Table S2 Surface elements composition of the prepared catalysts
| Sample | Element | Atomic fraction/% | Mass fraction/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Average | Area 1 | Area 2 | Area 3 | Average | ||
| MCZ-CP | O | 62.48 | 66.22 | 69.82 | 66.17 | 21.55 | 24.14 | 27.45 | 24.38 |
| Mn | 2.49 | 2.85 | 2.92 | 2.75 | 2.52 | 3.06 | 3.37 | 2.98 | |
| Zr | 15.70 | 11.73 | 10.37 | 12.60 | 26.47 | 20.91 | 19.94 | 22.44 | |
| Ce | 19.33 | 19.20 | 16.89 | 18.47 | 49.45 | 51.90 | 49.24 | 50.20 | |
| MCZ-IM | O | 60.16 | 63.95 | 68.43 | 64.18 | 21.21 | 24.43 | 26.76 | 24.13 |
| Mn | 6.91 | 8.73 | 4.92 | 6.86 | 7.16 | 9.81 | 5.66 | 7.54 | |
| Zr | 16.25 | 12.07 | 9.86 | 12.73 | 28.02 | 22.54 | 18.86 | 23.14 | |
| Ce | 16.68 | 15.25 | 16.80 | 16.24 | 43.61 | 43.22 | 48.72 | 45.18 | |
| MCZ-HB | O | 61.05 | 65.04 | 63.64 | 63.24 | 20.63 | 22.36 | 21.58 | 21.52 |
| Mn | 0.89 | 0.91 | 1.30 | 1.03 | 0.88 | 0.92 | 1.30 | 1.03 | |
| Zr | 19.84 | 11.62 | 12.91 | 14.79 | 32.79 | 19.54 | 21.41 | 24.58 | |
| Ce | 18.23 | 22.43 | 22.15 | 20.94 | 45.70 | 57.19 | 55.71 | 52.87 | |
| [1] | LIU S, FANG Z, WU X. CeO2-based materials for gasoline soot combustion: reaction mechanism and catalyst design. Journal of the Chinese Society of Rare Earths, 2022, 40(3): 351. |
| [2] |
LIU S, WU X D, TANG J, et al. An exploration of soot oxidation over CeO2-ZrO2 nanocubes: do more surface oxygen vacancies benefit the reaction? Catalysis Today, 2017, 281: 454.
DOI URL |
| [3] |
MATARRESE R. Catalytic materials for gasoline particulate filters soot oxidation. Catalysts, 2021, 11(8): 890.
DOI URL |
| [4] |
ZHAO M J, DENG J L, LIU J, et al. Roles of surface-active oxygen species on 3DOM cobalt-based spinel catalysts MxCo3-xO4 (M=Zn and Ni) for NOx-assisted soot oxidation. ACS Catalysis, 2019, 9(8): 7548.
DOI URL |
| [5] |
WANG X, JIN B F, FENG R X, et al. A robust core-shell silver soot oxidation catalyst driven by Co3O4: effect of tandem oxygen delivery and Co3O4-CeO2 synergy. Applied Catalysis B: Environmental, 2019, 250: 132.
DOI URL |
| [6] |
LI Y F, QIN T, WEI Y C, et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nature Communications, 2023, 14: 7149.
DOI PMID |
| [7] |
YU D, WANG L Y, ZHANG C L, et al. Alkali metals and cerium-modified La-co-based perovskite catalysts: facile synthesis, excellent catalytic performance, and reaction mechanisms for soot combustion. ACS Catalysis, 2022, 12(24): 15056.
DOI URL |
| [8] |
LIU S R, LUO S T, WU X D, et al. Application of silica-alumina as hydrothermally stable supports for Pt catalysts for acid-assisted soot oxidation. Rare Metals, 2023, 42(5): 1614.
DOI |
| [9] |
YU X H, REN Y, YU D, et al. Hierarchical porous K-OMS-2/ 3DOM-m Ti0.7Si0.3O2 catalysts for soot combustion: easy preparation, high catalytic activity, and good resistance to H2O and SO2. ACS Catalysis, 2021, 11(9): 5554.
DOI URL |
| [10] |
ZHU C X, DU S C, WANG S B, et al. PGM-free metal oxide nanoarray forests for water-promoted low-temperature soot oxidation. Applied Catalysis B: Environmental, 2024, 341: 123336.
DOI URL |
| [11] |
JIN B F, ZHAO B H, LIU S, et al. SmMn2O5 catalysts modified with silver for soot oxidation: dispersion of silver and distortion of mullite. Applied Catalysis B: Environmental, 2020, 273: 119058.
DOI URL |
| [12] |
LIU J X, YANG Z, ZHAI Y J, et al. High performance of PrMnO3 perovskite catalysts for low-temperature soot oxidation. Separation and Purification Technology, 2025, 354: 129227.
DOI URL |
| [13] |
YANG W N, WANG S M, LI K Z, et al. Highly selective α-Mn2O3 catalyst for cGPF soot oxidation: surface activated oxygen enhancement via selective dissolution. Chemical Engineering Journal, 2019, 364: 448.
DOI URL |
| [14] |
ZHAO Z, MA J, LI M, et al. Model Ag/CeO2 catalysts for soot combustion: roles of silver species and catalyst stability. Chemical Engineering Journal, 2022, 430: 132802.
DOI URL |
| [15] |
AWAD O I, MA X, KAMIL M, et al. Particulate emissions from gasoline direct injection engines: a review of how current emission regulations are being met by automobile manufacturers. Science of the Total Environment, 2020, 718: 137302.
DOI URL |
| [16] |
WANG X C, CHEN W H, HUANG Y H, et al. Advances in soot particles from gasoline direct injection engines: a focus on physical and chemical characterisation. Chemosphere, 2023, 311: 137181.
DOI URL |
| [17] |
KONTSES A, TRIANTAFYLLOPOULOS G, NTZIACHRISTOS L, et al. Particle number (PN) emissions from gasoline, diesel, LPG, CNG and hybrid-electric light-duty vehicles under real-world driving conditions. Atmospheric Environment, 2020, 222: 117126.
DOI URL |
| [18] |
NOSSOVA L, CARAVAGGIO G. Effect of dopants on soot oxidation over doped Ag/ZrO2 catalysts for catalyzed gasoline particulate filter. Catalysis Communications, 2023, 182: 106744.
DOI URL |
| [19] |
HERNÁNDEZ W Y, LOPEZ-GONZALEZ D, NTAIS S, et al. Silver-modified manganite and ferrite perovskites for catalyzed gasoline particulate filters. Applied Catalysis B: Environmental, 2018, 226: 202.
DOI URL |
| [20] |
YAO P, HUANG Y, JIAO Y, et al. Soot oxidation over Pt-loaded CeO2-ZrO2 catalysts under gasoline exhaust conditions: soot-catalyst contact efficiency and Pt chemical state. Fuel, 2023, 334: 126782.
DOI URL |
| [21] |
KUBO H, OHSHIMA Y, KATO S, et al. The effect of supported metal Species on soot oxidation over PGM/CeO2-ZrO2. Bulletin of the Chemical Society of Japan, 2024, 97(10): uoae092.
DOI URL |
| [22] |
LUO J B, ZHU X B, ZHONG Z W, et al. Enhanced catalytic soot oxidation over co-based metal oxides: effects of transition metal doping. Molecules, 2023, 29(1): 41.
DOI URL |
| [23] |
ZHANG P, MEI X L, ZHAO X C, et al. Boosting catalytic purification of soot particles over double perovskite-type La2-xKxNiCoO6 catalysts with an ordered macroporous structure. Environmental Science & Technology, 2021, 55(16): 11245.
DOI URL |
| [24] |
XIONG J X, ZHANG B J, LIANG Z F, et al. Highly reactive peroxide species promoted soot oxidation over an ordered macroporous Ce0.8Zr0.2O2 integrated catalyzed diesel particulate filter. Environmental Science & Technology, 2024, 58(18): 8096.
DOI URL |
| [25] |
HE J S, YAO P, QIU J, et al. Enhancement effect of oxygen mobility over Ce0.5Zr0.5O2 catalysts doped by multivalent metal oxides for soot combustion. Fuel, 2021, 286: 119359.
DOI URL |
| [26] |
DENG J, LI S S, XIONG L, et al. Preparation of nanostructured CeO2-ZrO2-based materials with stabilized surface area and their catalysis in soot oxidation. Applied Surface Science, 2020, 505: 144301.
DOI URL |
| [27] |
WANG L M, ZHAO N R, YIN X Y, et al. Highlights on the key roles of interfaces between CeO2-based oxide and perovskite (LaMnO3/LaFeO3) in creating active oxygen species for soot oxidation. Fuel, 2024, 356: 129444.
DOI URL |
| [28] |
ZHENG C L, MAO D J, XU Z Y, et al. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation. Journal of Catalysis, 2022, 411: 122.
DOI URL |
| [29] |
XIONG L, YAO P, LIU S, et al. Soot oxidation over CeO2-ZrO2 based catalysts: the influence of external surface and low- temperature reducibility. Molecular Catalysis, 2019, 467: 16.
DOI URL |
| [30] |
MISHRA U K, CHANDEL V S, SINGH O P, et al. Synthesis of CeO2 and Zr-doped CeO2 (Ce1-xZrxO2) catalyst by green synthesis for soot oxidation activity. Arabian Journal for Science and Engineering, 2023, 48(1): 771.
DOI |
| [31] |
LIU S, WU X D, WENG D, et al. Ceria-based catalysts for soot oxidation: a review. Journal of Rare Earths, 2015, 33(6): 567.
DOI URL |
| [32] |
LI Y F, QIN T, XIONG J, et al. Mn-modified near-surface atomic structure of CeO2 nanorods for promoting catalytic oxidation of auto-exhaust carbon particles. Chemical Engineering Science, 2023, 282: 119309.
DOI URL |
| [33] |
ZHAO H, ZHOU X X, WANG M, et al. Highly active MnOx-CeO2 catalyst for diesel soot combustion. RSC Advances, 2017, 7(6): 3233.
DOI URL |
| [34] |
ALINEZHADCHAMAZKETI A, KHODADADI A A, MORTAZAVI Y, et al. Catalytic evaluation of promoted CeO2-ZrO2 by transition, alkali, and alkaline-earth metal oxides for diesel soot oxidation. Journal of Environmental Sciences, 2013, 25(12): 2498.
DOI URL |
| [35] | XING L L, YANG Y X, CAO C M, et al. Decorating CeO2 nanoparticles on Mn2O3 nanosheets to improve catalytic soot combustion. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16544. |
| [36] |
YANG Y, FANG J, MENG Z W, et al. Catalytic activity and influence factors of Mn-Ce mixed oxides by hydrothermal method on diesel soot combustion. Molecular Catalysis, 2022, 524: 112334.
DOI URL |
| [37] |
SUN Y, FANG S Y, XU J C, et al. Unveiling the surface chemical reactions during multi-phase catalytic oxidation of soot on nanoengineering/interfacing/doping-prepared Mn-CeO2 catalysts using TG-MS and operando DRIFTS-MS. Langmuir, 2023, 39(44): 15773.
DOI URL |
| [38] |
ZHAO H, LI H C, PAN Z F, et al. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Applied Catalysis B: Environmental, 2020, 268: 118422.
DOI URL |
| [39] |
LI S S, DENG J, WANG J L, et al. Effects of thermal treatment conditions on redox properties of ceria-zirconia materials. Journal of Rare Earths, 2023, 41(12): 1969.
DOI URL |
| [40] |
YAO P, HE J S, JIANG X, et al. Factors determining gasoline soot abatement over CeO2-ZrO2-MnOx catalysts under low oxygen concentration condition. Journal of the Energy Institute, 2020, 93(2): 774.
DOI URL |
| [41] |
ZHANG H L, WANG J L, ZHANG Y H, et al. A study on H2-TPR of Pt/Ce0.27Zr0.73O2 and Pt/Ce0.27Zr0.70La0.03Ox for soot oxidation. Applied Surface Science, 2016, 377: 48.
DOI URL |
| [42] | WANG S N, WANG J L, HUA W B, et al. Designed synthesis of Zr-based ceria-zirconia-neodymia composite with high thermal stability and its enhanced catalytic performance for Rh-only three- way catalyst. Catalysis Science & Technology, 2016, 6(20): 7437. |
| [43] |
HE H, LIN X T, LI S J, et al. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Applied Catalysis B: Environmental, 2018, 223: 134.
DOI URL |
| [44] |
WANG H L, LUO S T, ZHANG M S, et al. Roles of oxygen vacancy and Ox- in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts. Journal of Catalysis, 2018, 368: 365.
DOI URL |
| [45] |
XIONG J, WU Q Q, MEI X L, et al. Fabrication of spinel-type PdxCo3-xO4 binary active sites on 3D ordered meso-macroporous Ce-Zr-O2 with enhanced activity for catalytic soot oxidation. ACS Catalysis, 2018, 8(9): 7915.
DOI URL |
| [46] |
KHATUN R, PAL R S, SHOEB M A, et al. Generation of active oxygen species by CO2 dissociation over defect-rich Ni-Pt/CeO2 catalyst for boosting methane activation in low-temperature dry reforming: experimental and theoretical study. Applied Catalysis B: Environmental, 2024, 340: 123243.
DOI URL |
| [47] |
LIN X T, LI S J, HE H, et al. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Applied Catalysis B: Environmental, 2018, 223: 91.
DOI URL |
| [48] |
ZHANG Z R, KANG R N, YI X K, et al. Migration roles of different oxygen species over Cu/CeO2 for propane and soot combustion. Separation and Purification Technology, 2024, 349: 127820.
DOI URL |
| [49] |
XU J W, ZHANG Y, XU X L, et al. Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: the effect of phase structures, superoxide anions, and alkalinity on the reactivity. ACS Catalysis, 2019, 9(5): 4030.
DOI URL |
| [50] |
SUBBOTINA I R, BARSUKOV D V. Direct evidence of the key role of UV-formed peroxide species in photocatalytic gas-solid oxidation in air on anatase TiO2 particles. Physical Chemistry Chemical Physics, 2020, 22(4): 2200.
DOI URL |
| [51] | ZHANG M Y, DUAN X L, GAO Y, et al. Tuning oxygen vacancies in oxides by configurational entropy. ACS Applied Materials & Interfaces, 2023, 15(39): 45774. |
| [52] | MAO H F, XU M L, LI S J, et al. Accelerating surface lattice oxygen activation of Pt/TiO2-x by modulating the interface electron interaction for efficient photocatalytic toluene oxidation. ACS ES&T Engineering, 2023, 3(11): 1851. |
| [53] |
LOU D M, SONG G F, XU K W, et al. The oxidation performance of a carbon soot catalyst based on the Pt-Pd synergy effect. Energies, 2024, 17(7): 1737.
DOI URL |
| [1] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [2] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [3] | LI Jianjun, CHEN Fangming, ZHANG Lili, WANG Lei, ZHANG Liting, CHEN Huiwen, XUE Changguo, XU Liangji. Peroxymonosulfate Activation by CoFe2O4/MgAl-LDH Catalyst for the Boosted Degradation of Antibiotic [J]. Journal of Inorganic Materials, 2025, 40(4): 440-448. |
| [4] | XIN Zhenyu, GUO Ruihua, WUREN Tuoya, WANG Yan, AN Shengli, ZHANG Guofang, GUAN Lili. Pt-Fe/GO Nanocatalysts: Preparation and Electrocatalytic Performance on Ethanol Oxidation [J]. Journal of Inorganic Materials, 2025, 40(4): 379-387. |
| [5] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [6] | LI Xueru, MA Zhejie, GUO Yujie, LI Ping. Influence of Support Characteristics on Coverage of Ionomer and Oxygen Reduction Performance for Pt/C Catalysts [J]. Journal of Inorganic Materials, 2025, 40(12): 1395-1404. |
| [7] | ZHAO Lijuan, TAN Zhe, ZHANG Xiaoguang, JIANG Guosai, TAO Ran, PAN De’an. Numerical Simulation of Particle Classification for Spent Hydrogenation Catalyst [J]. Journal of Inorganic Materials, 2025, 40(12): 1387-1394. |
| [8] | LIU Huilai, LI Zhihao, KONG Defeng, CHEN Xing. Preparation of FePc/MXene Composite Cathode and Electro-Fenton Degradation of Sulfadimethoxine [J]. Journal of Inorganic Materials, 2025, 40(1): 61-69. |
| [9] | LI Na, CAO Ruixiao, WEI Jin, ZHOU Han, XIAO Hongmei. Performance and Influencing Factors of Iron-based Catalyst for Ortho to Para Hydrogen Conversion [J]. Journal of Inorganic Materials, 2025, 40(1): 47-52. |
| [10] | LIAN Minli, SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin. Supported Ni Catalysts from Ni-Mg-Al Hydrotalcite-like Compounds:Preparation and Catalytic Performance for Ammonia Decomposition [J]. Journal of Inorganic Materials, 2025, 40(1): 53-60. |
| [11] | LIU Lei, GUO Ruihua, WANG Li, WANG Yan, ZHANG Guofang, GUAN Lili. Oxygen Reduction Reaction on Pt3Co High-index Facets by Density Functional Theory [J]. Journal of Inorganic Materials, 2025, 40(1): 39-46. |
| [12] | JIN Yuxiang, SONG Erhong, ZHU Yongfu. First-principles Investigation of Single 3d Transition Metals Doping Graphene Vacancies for CO2 Electroreduction [J]. Journal of Inorganic Materials, 2024, 39(7): 845-852. |
| [13] | YE Zibin, ZOU Gaochang, WU Qiwen, YAN Xiaomin, ZHOU Mingyang, LIU Jiang. Preparation and Performances of Tubular Cone-shaped Anode-supported Segmented-in-series Direct Carbon Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2024, 39(7): 819-827. |
| [14] | ZHANG Wenyu, GUO Ruihua, YUE Quanxin, HUANG Yarong, ZHANG Guofang, GUAN Lili. High-entropy Phosphide Bifunctional Catalyst: Preparation and Performance of Efficient Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1265-1274. |
| [15] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||