
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (7): 793-799.DOI: 10.15541/jim20220658
• RESEARCH ARTICLE • Previous Articles Next Articles
MENG Bo1( ), XIAO Gang2, WANG Xiuli1, TU Jiangping1, GU Changdong1(
), XIAO Gang2, WANG Xiuli1, TU Jiangping1, GU Changdong1( )
)
Received:2022-11-07
Revised:2022-12-04
Published:2022-12-16
Online:2022-12-27
Contact:
GU Changdong, associate professor. E-mail: cdgu@zju.edu.cnAbout author:MENG Bo (1998-), female, Master candidate. E-mail: 22060231@zju.edu.cn
Supported by:CLC Number:
MENG Bo, XIAO Gang, WANG Xiuli, TU Jiangping, GU Changdong. Ionic Thermal Synthesis and Reversible Heat Storage Performance of Manganese-based Oxides[J]. Journal of Inorganic Materials, 2023, 38(7): 793-799.
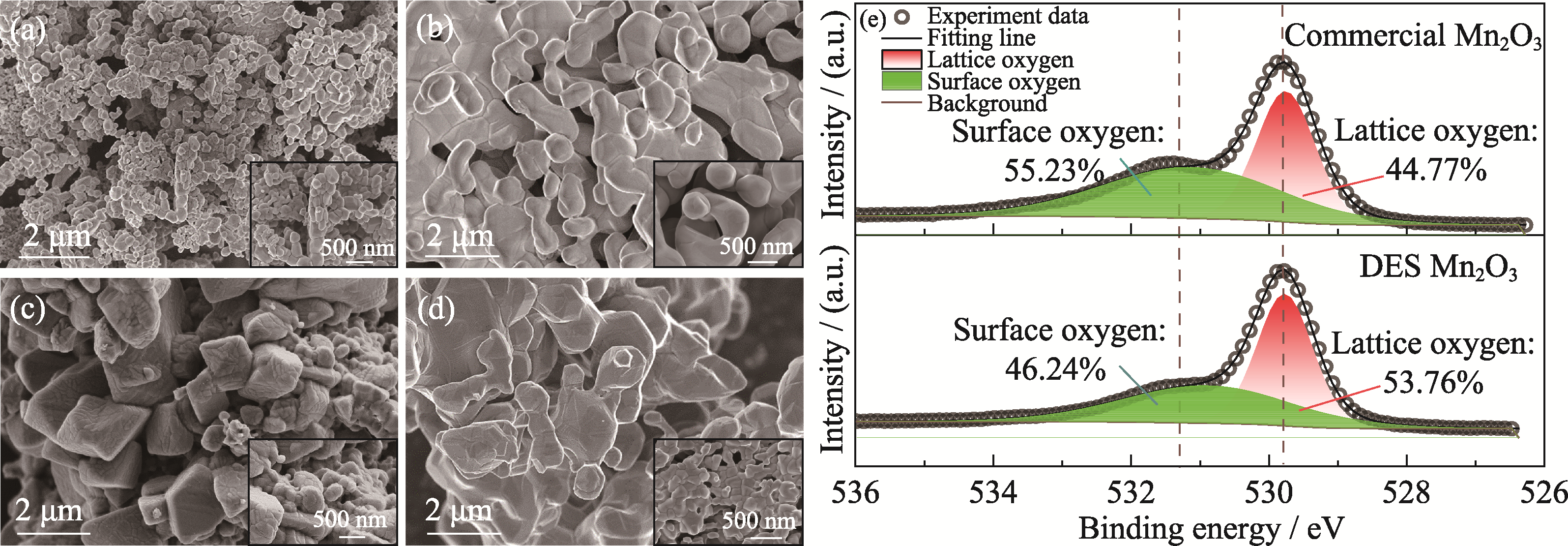
Fig. 2 (a-d) SEM images of samples before and after cycling, and (e) O1s XPS spectra of Mn2O3 before cycling (a) Commercial Mn2O3 before cycling; (b) Commercial Mn2O3 after cycling; (c) DES Mn2O3 before cycling; (d) DES Mn2O3 after cycling
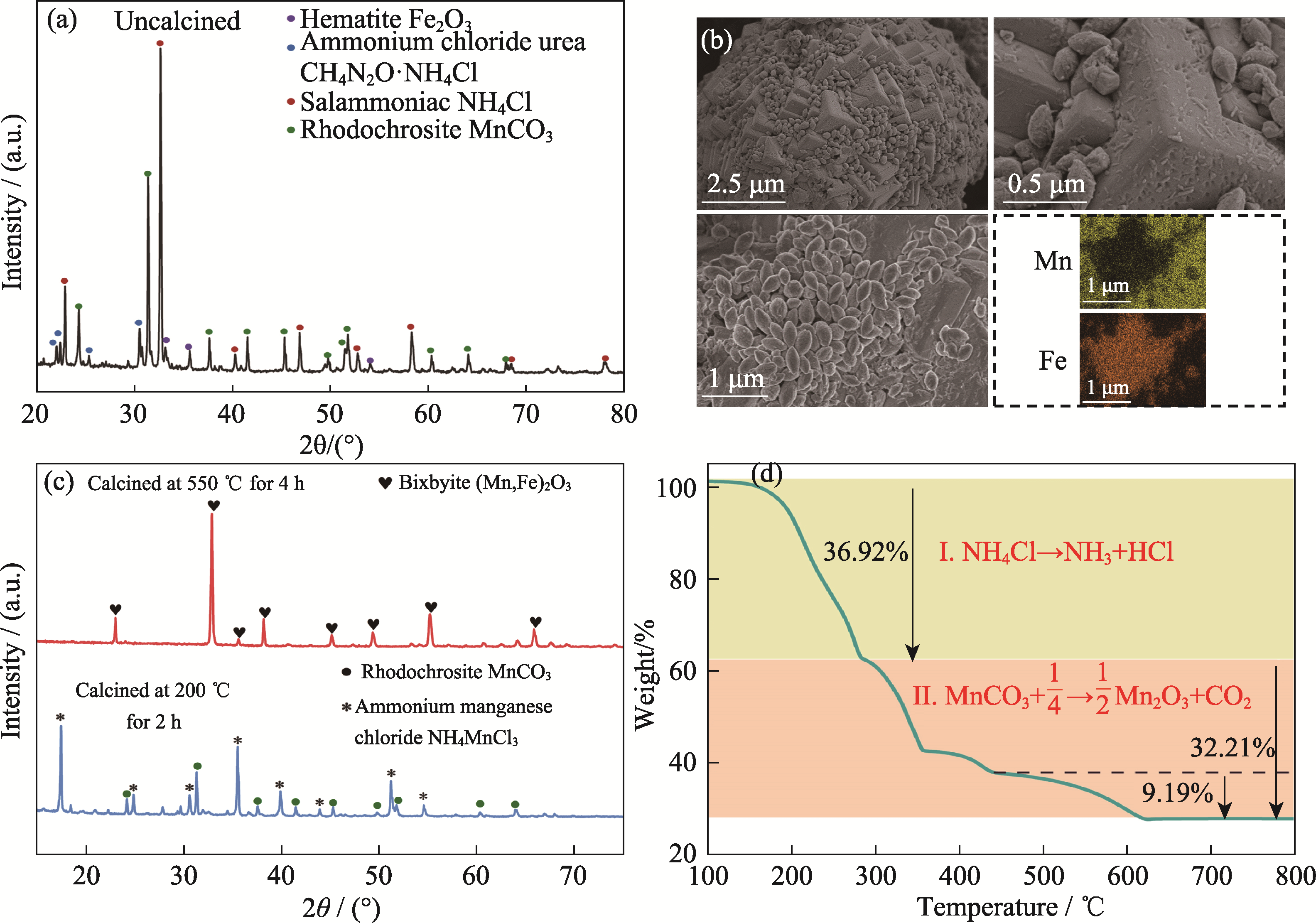
Fig. 8 Characterization of precursors of manganese-based oxide synthesized by DES (a) XRD pattern; (b) SEM-EDS images; (c) XRD patterns under different heat-treatments; (d) TG curve
| [1] |
PFENNINGER S, GAUCHÉ P, LILLIESTAM J, et al. Potential for concentrating solar power to provide baseload and dispatchable power. Nature Climate Change, 2014, 4(8): 689.
DOI |
| [2] |
PRASAD J S, MUTHUKUMAR P, DESAI F, et al. A critical review of high-temperature reversible thermochemical energy storage systems. Applied Energy, 2019, 254: 113733.
DOI URL |
| [3] |
LANCHI M, TURCHETTI L, SAU S, et al. A discussion of possible approaches to the integration of thermochemical storage systems in concentrating solar power plants. Energies, 2020, 13(18): 4940.
DOI URL |
| [4] |
ANDRÉ L, ABANADES S, FLAMANT G, et al. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renewable and Sustainable Energy Reviews, 2016, 64: 703.
DOI URL |
| [5] |
BLOCK T, SCHMUCKER M. Metal oxides for thermochemical energy storage: a comparison of several metal oxide systems. Solar Energy, 2016, 126: 195.
DOI URL |
| [6] |
CARRILLO A J, GONZALEZ-AGUILAR J, ROMERO M, et al. Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials. Chemical Reviews. 2019, 119(7): 4777.
DOI PMID |
| [7] |
WU S, ZHOU C, DOROODCHI E, et al. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Conversion and Management, 2018, 168: 421.
DOI URL |
| [8] | BIELSA D, ZAKI A, FAIK A, et al. Efficiency improvement of Mn2O3/Mn3O4 redox reaction by means of different operation strategies. AIP Conference Proceedings, 2019, 2126: 210001. |
| [9] |
CARRILLO A J, SERRANO D P, PIZARRO P, et al. Thermochemical heat storage based on the Mn2O3/Mn3O4 redox couple: influence of the initial particle size on the morphological evolution and cyclability. Journal of Materials Chemistry A, 2014, 2(45): 19435.
DOI URL |
| [10] | CARRILLO A J, SERRANO D P, PIZARRO P, et al. Design of efficient Mn-based redox materials for thermochemical heat storage at high temperatures. AIP Conference Proceedings, 2016, 1734: 050009. |
| [11] |
BIELSA D, ZAKI A, ARIAS P L, et al. Improving the redox performance of Mn2O3/Mn3O4 pair by Si doping to be used as thermochemical energy storage for concentrated solar power plants. Solar Energy, 2020, 204: 144.
DOI URL |
| [12] |
CHEN X, KUBOTA M, YAMASHITA S, et al. Exploring Cu-based spinel/delafossite couples for thermochemical energy storage at medium-high temperature. ACS Applied Energy Materials, 2021, 4(7): 7242.
DOI URL |
| [13] |
ANDRÉ L, ABANADES S, CASSAYRE L, et al. Experimental investigation of Co-Cu, Mn-Co, and Mn-Cu redox materials applied to solar thermochemical energy storage. ACS Applied Energy Materials, 2018, 1(7): 3385.
DOI URL |
| [14] |
HLONGWA N W, SASTRE D, IWUOHA E, et al. Exploring the thermochemical heat storage capacity of AMn2O4(A=Li or Cu) spinels. Solid State Ionics, 2018, 320: 316.
DOI URL |
| [15] |
ABAD A, MENDIARA T, IZQUIERDO M T, et al. Evaluation of the redox capability of manganese-titanium mixed oxides for thermochemical energy storage and chemical looping processes. Fuel Processing Technology, 2021, 211: 106579.
DOI URL |
| [16] |
RANDHIR K, KING K, RHODES N, et al. Oxidation kinetics of magnesium-manganese oxides for high-temperature thermochemical energy storage. Energy Technology, 2020, 8(10): 2000063.
DOI URL |
| [17] |
CARRILLO A J, PIZARRO P, CORONADO J M, et al. Assessing Cr incorporation in Mn2O3/Mn3O4 redox materials for thermochemical heat storage applications. Journal of Energy Storage, 2020, 33: 102028.
DOI URL |
| [18] |
WOKON M, KOHZER A, LINDER M. Investigations on thermochemical energy storage based on technical grade manganese-iron oxide in a lab-scale packed bed reactor. Solar Energy, 2017, 153: 200.
DOI URL |
| [19] |
AL-SHANKITI I A, EHRHART B D, WARD B J, et al. Particle design and oxidation kinetics of iron manganese oxide redox materials for thermochemical energy storage. Solar Energy, 2019, 183: 17.
DOI URL |
| [20] |
WANG B, LI L, SCHAFER F, et al. Thermal reduction of iron-manganese oxide particles in a high-temperature packed-bed solar thermochemical reactor. Chemical Engineering Journal, 2021, 412: 128255.
DOI URL |
| [21] |
GE X, GU C D, WANG X L, et al. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision. Journal of Materials Chemistry A, 2017, 5(18): 8209.
DOI URL |
| [22] |
LIU Y, CHI X, HANQ, et al. α-MnO2 nanofibers/carbon nanotubes hierarchically assembled microspheres: approaching practical applications of high-performance aqueous Zn-ion batteries. Journal of Power Sources, 2019, 443: 227244.
DOI URL |
| [23] |
ZHANG Z, YU J, ZHANG J, et al. Tailored metastable Ce-Zr oxides with highly distorted lattice oxygen for accelerating redox cycles. Chemical Science, 2018, 9(13): 3386.
DOI PMID |
| [24] |
XIANG D, GU C D, XU H R, et al. Self-assembled structure evolution of Mn-Fe oxides for high temperature thermochemical energy storage. Small, 2021, 17(29): 2101524.
DOI URL |
| [25] | 张杰, 唐定国, 刘浩文, 等. 碳酸锰高温分解制备三氧化二锰研究. 山东化工, 2013, 42(4): 1. |
| [26] |
NOUR E M, TELEB S M, AL-KHSOSY N A, et al. A novel method for the synthesis of metal carbonates. I.synthesis and infrared spectrum of manganese carbonate, MnCO3·H2O, formed by the reaction of urea with manganese(II) salts. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 1997, 27(4): 505.
DOI URL |
| [27] |
XIONG Q Q, TU J P, GE X, et al. One-step synthesis of hematite nanospindles from choline chloride/urea deep eutectic solvent with highly powerful storage versus lithium. Journal of Power Sources, 2015, 274: 1.
DOI URL |
| [1] | LI Meng-Xia, LU Yue, WANG Li-Bin, HU Xian-Luo. Controlled Synthesis of Core-shell Structured Mn3O4@ZnO Nanosheet Arrays for Aqueous Zinc-ion Batteries [J]. Journal of Inorganic Materials, 2020, 35(1): 86-92. |
| [2] | YANG Ying, ZHANG Zheng, GAO Jing, LIN Ze-Hua, YAN Jing-Yuan, GUO Xue-Yi. Deep Eutectic Solvent Based Polymer Electrolyte for Dye-sensitized Solar Cells [J]. Journal of Inorganic Materials, 2017, 32(1): 25-32. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||