无机材料学报 ›› 2023, Vol. 38 ›› Issue (12): 1441-1448.DOI: 10.15541/jim20230198 CSTR: 32189.14.10.15541/jim20230198
所属专题: 【生物材料】抗菌与肿瘤治疗(202506); 【生物材料】骨骼与齿类组织修复(202506)
杜佳恒1,2( ), 范鑫丽3,4, 肖东琴2, 尹一然1, 李忠1, 贺葵1, 段可1(
), 范鑫丽3,4, 肖东琴2, 尹一然1, 李忠1, 贺葵1, 段可1( )
)
收稿日期:2023-04-18
修回日期:2023-06-03
出版日期:2023-10-15
网络出版日期:2023-10-15
通讯作者:
段 可, 教授. E-mail: keduan@swmu.edu.cn作者简介:杜佳恒(1997-), 男, 硕士研究生. E-mail: dujiaheng1011@163.com
基金资助:
DU Jiaheng1,2( ), FAN Xinli3,4, XIAO Dongqin2, YIN Yiran1, LI Zhong1, HE Kui1, DUAN Ke1(
), FAN Xinli3,4, XIAO Dongqin2, YIN Yiran1, LI Zhong1, HE Kui1, DUAN Ke1( )
)
Received:2023-04-18
Revised:2023-06-03
Published:2023-10-15
Online:2023-10-15
Contact:
DUAN Ke, professor. E-mail: keduan@swmu.edu.cnAbout author:DU Jiaheng (1997-), male, Master candidate. E-mail: dujiaheng1011@163.com
Supported by:摘要:
骨科钛内置物存在感染的风险, 需要开发具有抗菌性、生物相容性且不易产生耐药性的表面涂层。通过电泳沉积15、30、45、60 s在微弧氧化(MAO)的钛表面制备了4组纳米氧化镁(MgO)涂层。MgO颗粒在MAO表面形成均匀涂层, 覆盖率随电泳时间延长。与金黄色葡萄球菌共培养6 h后, 4组样品抗菌率分别为1%、69%、83%、84%; 共培养24 h后抗菌率分别为81%、86%、89%、98%。显微观察发现MgO沉积样品表面黏附细菌密度、活细菌比例均随沉积时间延长而减少。与小鼠成骨细胞共培养1 d后, 4组样品存活率(相对空白孔板中所接种细胞)分别为108%、89%、53%、27%, 5 d后分别为139%、117%、112%、66%。荧光显微观察发现MAO样品表面未见死细胞, 而MgO沉积样品表面死细胞比例随沉积时间延长而增加, 但在实验周期(5 d)内均<5%。本研究表明电泳沉积30 s制备的MgO涂层具有良好的体外抗菌性和生物相容性。
中图分类号:
杜佳恒, 范鑫丽, 肖东琴, 尹一然, 李忠, 贺葵, 段可. 电泳沉积制备微弧氧化钛表面氧化镁涂层及其生物学性能[J]. 无机材料学报, 2023, 38(12): 1441-1448.
DU Jiaheng, FAN Xinli, XIAO Dongqin, YIN Yiran, LI Zhong, HE Kui, DUAN Ke. Electrophoretic Coating of Magnesium Oxide on Microarc-oxidized Titanium and Its Biological Properties[J]. Journal of Inorganic Materials, 2023, 38(12): 1441-1448.
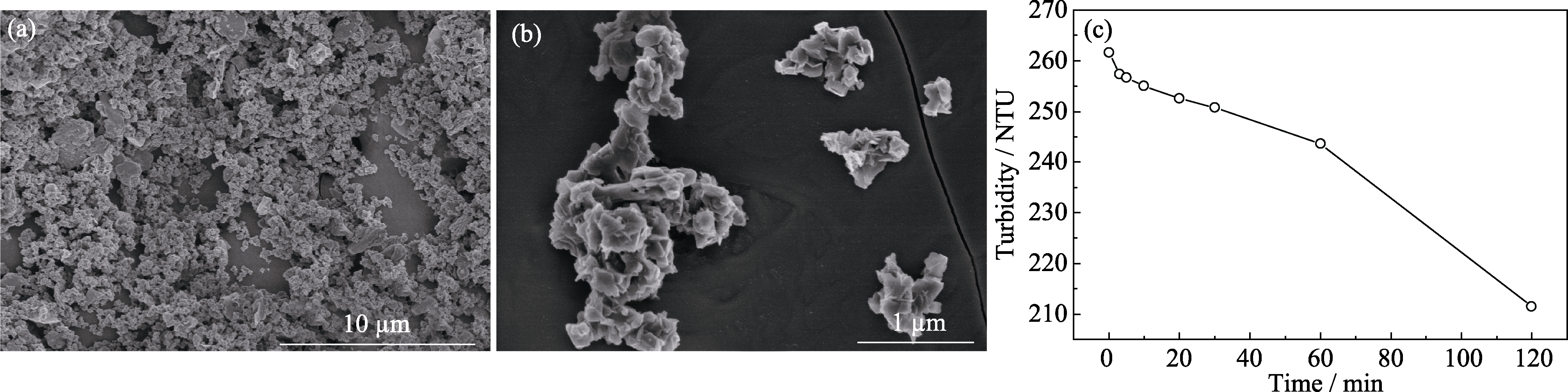
图1 (a, b) 纳米MgO的低、高倍SEM形貌及(c)纳米MgO/丙酮悬浊液浊度随时间的变化曲线
Fig. 1 (a, b) Low and high-power SEM images of nano-MgO powder used and (c) variation of turbidity of nano-MgO/acetone suspension with time
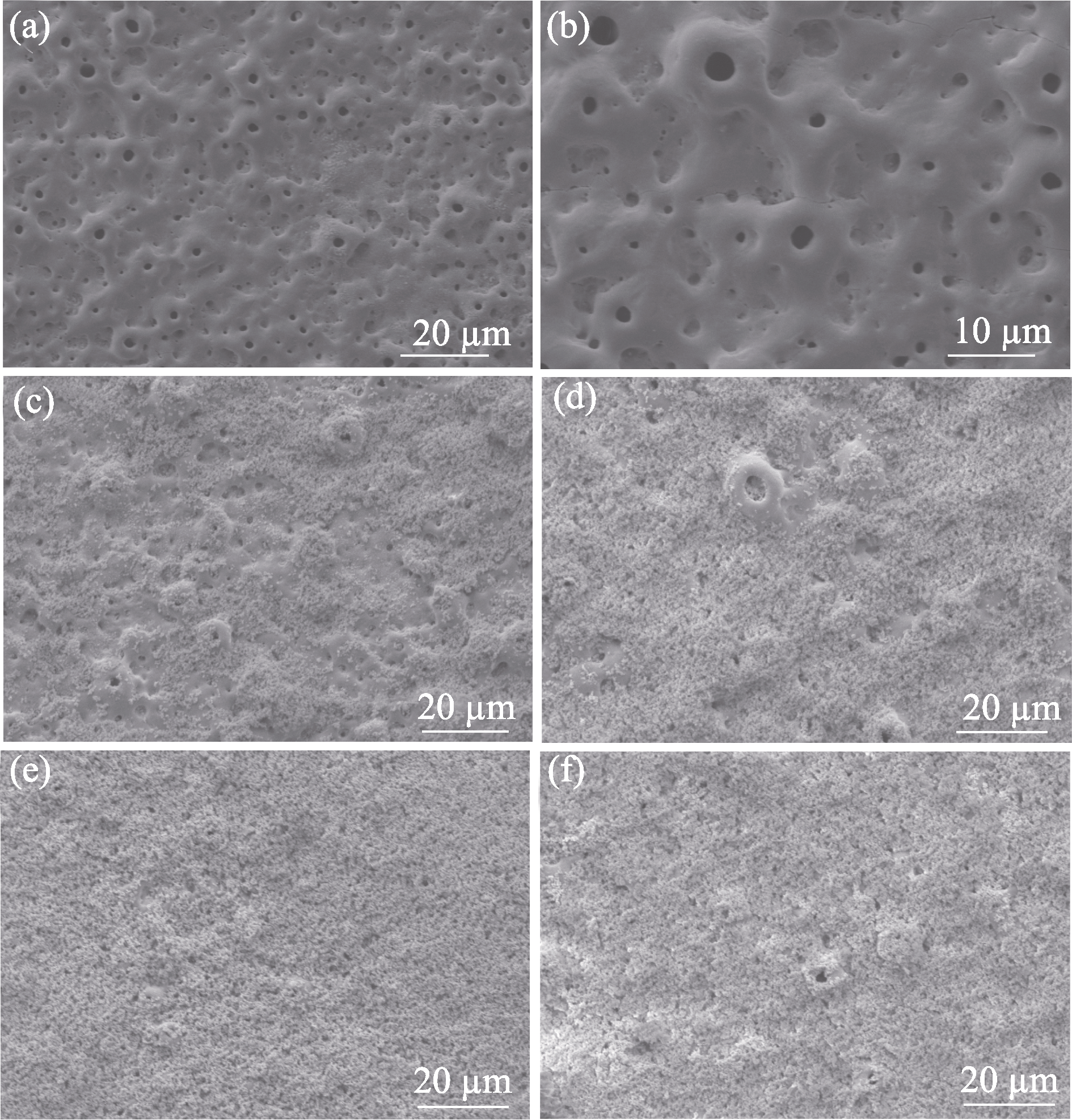
图2 样品的SEM表面形貌
Fig. 2 SEM images of samples MAO at low (a) and high (b) magnification ratios; (c) MAO-MgO15; (d) MAO-MgO30; (e) MAO-MgO45; (f) MAO-MgO60

图7 (a)各组样品与细菌共培养24 h后冲洗液涂板形成菌落的典型照片及(b)共培养后的抗菌率
Fig. 7 (a) Representative photographs of colonies formed on samples after co-cultured with S. aureus for 24 h, and (b) antibacterial rates Numbers 2, 3 and 4 indicating MAO vs. MAO-MgO30, MAO-MgO45, MAO-MgO60, respectively (p<0.05)

图8 各组样品与细菌共培养24 h并活/死染色后的荧光显微照片
Fig. 8 Micrographs of samples after Live/Dead fluorescent staining on co-cultured S. aureus for 24 h Red pixels: Dead S. aureus cells; Green pixels: Alive S. aureus cells; All scale bars: 100 μm

图9 样品接种细菌培养24 h后表面SEM形貌
Fig. 9 SEM micrographs of samples (a) MAO and (b-e) MAO-MgO15 to MAO-MgO60 after co-culture with S. aureus for 24 h; Red arrows pointing to S. aureus cells
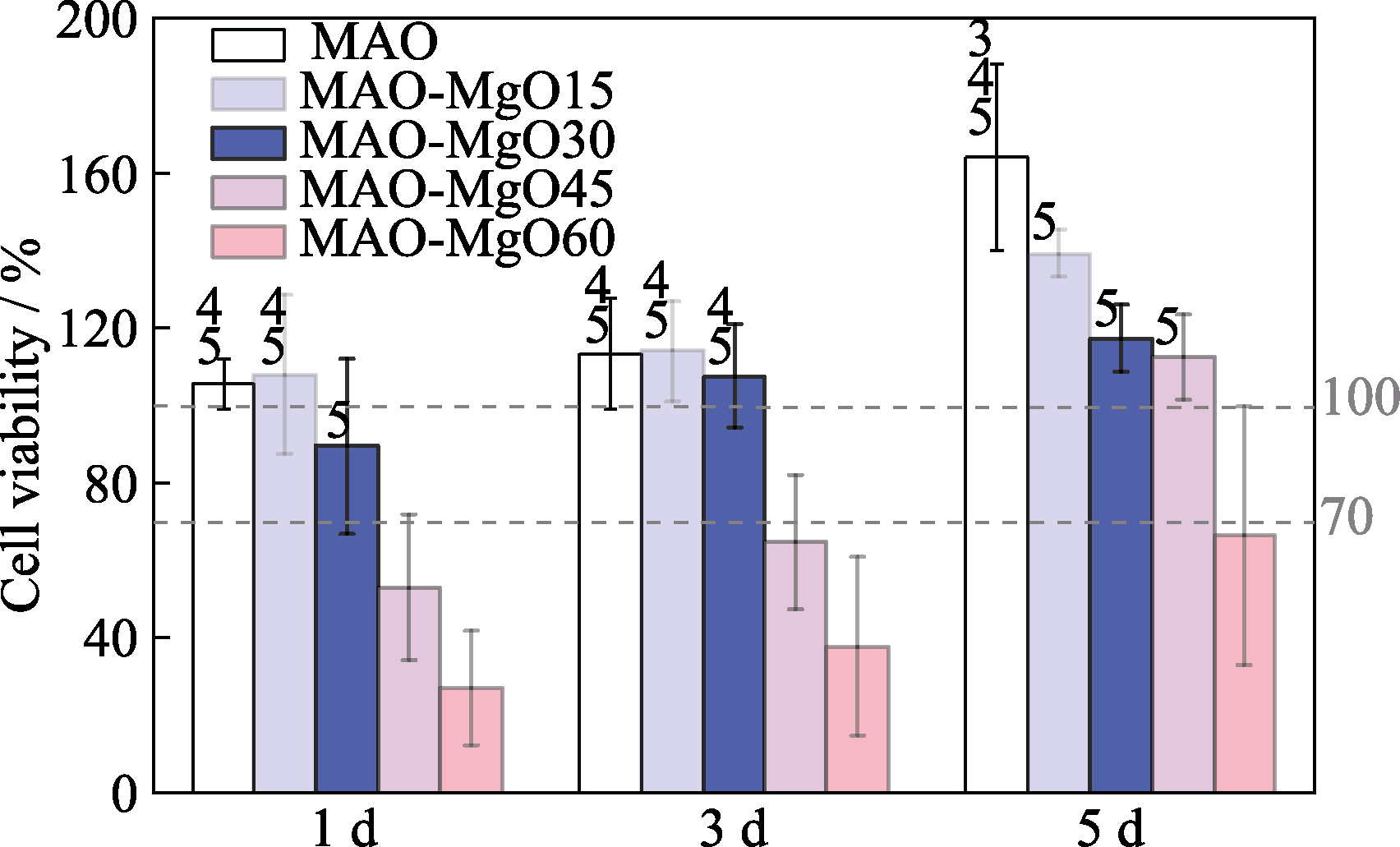
图10 细胞接种在样品表面培养1~5 d后的相对存活率
Fig. 10 Viability of rat osteoblasts after co-culture on samples for 1, 3 and 5 d Numbers 3, 4 and 5 indicating MAO vs. MAO-MgO30, MAO-MgO45, MAO-MgO60, respectively (p<0.05)
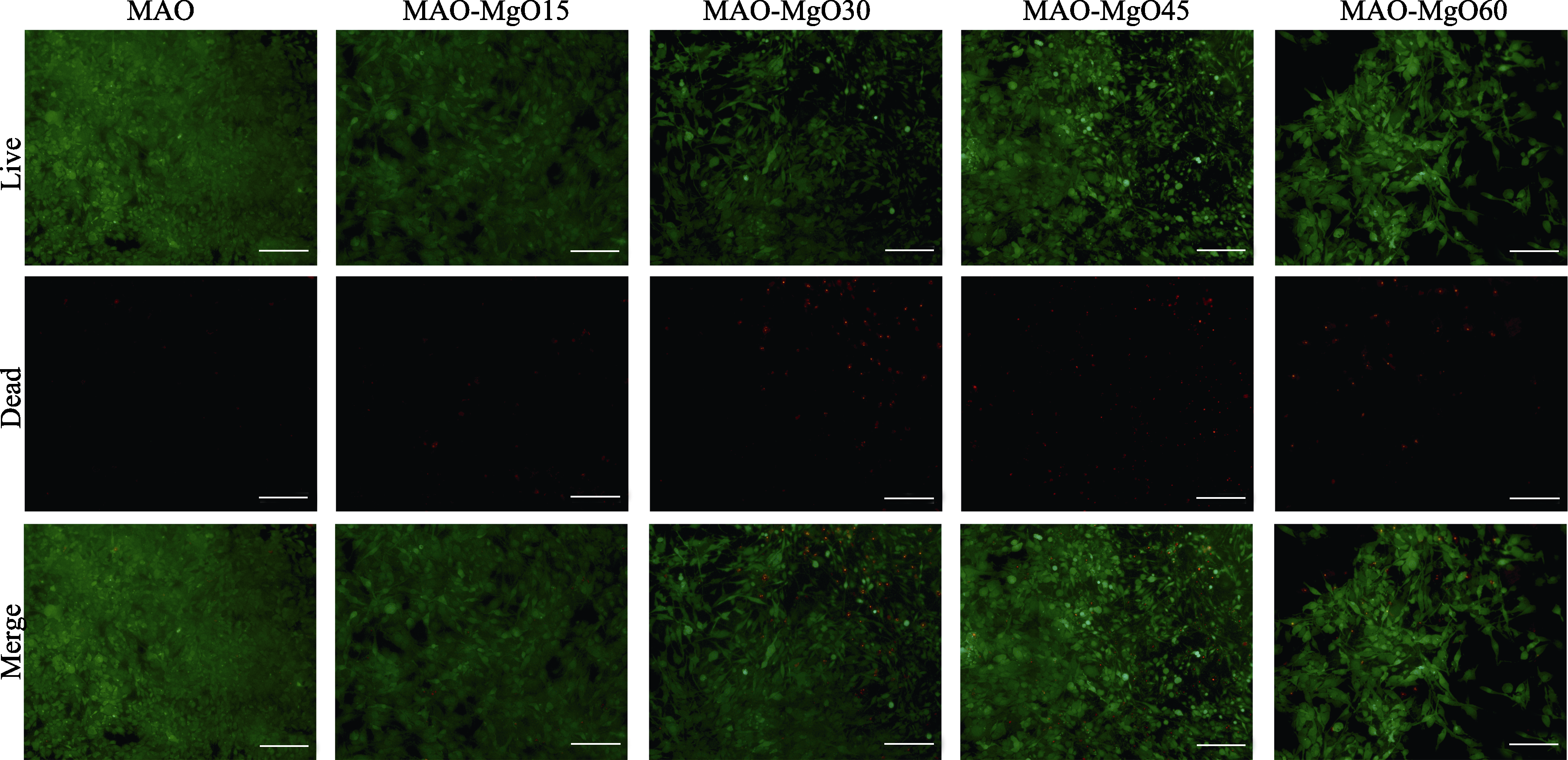
图11 各组样品与细胞共培养5 d并活/死染色后的荧光显微照片
Fig. 11 Images of samples after Live/Dead fluorescent staining on co-cultured rat osteoblasts for 5 d Red pixels: Dead cells; Green pixels: Alive cells; All scale bars: 100 μm
| [1] |
POTAPOVA I. Functional imaging in diagnostic of orthopedic implant-associated infections. Diagnostics, 2013, 3(4): 356.
DOI PMID |
| [2] |
MALIZOS K, BLAUTH M, DANITA A, et al. Fast-resorbable antibiotic- loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: a multicenter randomized controlled trial. Journal of Orthopaedics and Traumatology, 2017, 18(2): 159.
DOI URL |
| [3] |
CAO H, MENG F, LIU X, et al. Antimicrobial activity of tantalum oxide coatings decorated with Ag nanoparticles. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2016, 34(4): 04C102.
DOI URL |
| [4] | OLSEN I. Biofilm-specific antibiotic tolerance and resistance. European Journal of Clinical Microbiology & Infectious Diseases, 2015, 34(5): 877. |
| [5] |
SCHINS R P F, KNAAPEN A M. Genotoxicity of poorly soluble particles. Inhalation Toxicology, 2007, 19(sup1): 189.
DOI URL |
| [6] |
PALACIOS-HERNANDEZ T, DIAZ-DIESTRA DM, NGUYEN A K, et al. Cytotoxicity, cellular uptake and apoptotic responses in human coronary artery endothelial cells exposed to ultrasmall superparamagnetic iron oxide nanoparticles. Journal of Applied Toxicology, 2020, 40(7): 918.
DOI URL |
| [7] | WANG Y, YU H, CHEN C, et al. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Materials & Design, 2015, 8(5): 640. |
| [8] | XUE T, ATTARILAR S, LIU S, et al. Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: thematic review. Frontiers in Bioengineering and Biotechnology, 2020, 8: 603072. |
| [9] |
AL-AHMAD A, WIEDMANN-AL-AHMAD M, FACKLER A, et al. In vivo study of the initial bacterial adhesion on different implant materials. Archives of Oral Biology, 2013, 58(9): 1139.
DOI URL |
| [10] | AL-AHMAD A, WIEDMANN-AL-AHMAD M, FAUST J, et al. Biofilm formation and composition on different implant materials in vivo. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2010, 95(1): 101. |
| [11] | CHEN Y, SHENG W, LIN J, et al. Magnesium oxide nanoparticle coordinated phosphate-functionalized chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. ACS Applied Materials & Interfaces, 2022, 14(6): 7592. |
| [12] |
CHEN R, CHEN H B, XUE P P, et al. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. Journal of Materials Chemistry B, 2021, 9(4): 1107.
DOI PMID |
| [13] | LUQUE-AGUDO V, FERNÁNDEZ-CALDERÓN M C, PACHA- OLIVENZA M A, et al. The role of magnesium in biomaterials related infections. Colloids and Surfaces B: Biointerfaces, 2020, 191: 110996. |
| [14] | NGUYEN N Y T, GRELLING N, WETTELAND C L, et al. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Scientific Reports, 2018, 8: 16260. |
| [15] | COELHO C C, PADRÃO T, COSTA L, et al. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Scientific Reports, 2020, 10: 19098. |
| [16] | BOCCACCINI A R, KEIM S, MA R, et al. Electrophoretic deposition of biomaterials. Journal of the Royal Society Interface, 2010, 7(suppl_5): S581. |
| [17] |
BRUCHIEL-SPANIER N, BETSIS S, NAIM G, et al. Electrochemical and electrophoretic coatings of medical implants by nanomaterials. Journal of Solid State Electrochemistry, 2022, 26(9): 1871.
DOI |
| [18] |
HICKEY D J, MUTHUSAMY D, WEBSTER T J. Electrophoretic deposition of MgO nanoparticles imparts antibacterial properties to poly-L-lactic acid for orthopedic applications. Journal of Biomedical Materials Research Part A, 2017, 105(11): 3136.
DOI PMID |
| [19] | LIN Y J, LI D Q, WANG G, et al. Preparation and bactericidal property of MgO nanoparticles on gamma-Al2O3. Journal Materials Science: Materials in Medcine, 2005, 16(1): 53. |
| [20] | AL-SHARABI A, SADA'A KSS, AL-OSTA A, et al. Structure, optical properties and antimicrobial activities of MgO-BiCrO nanocomposites prepared via solvent-deficient method. Scientific Reports, 2022, 12: 10647. |
| [21] | LI X, HONG X, YANG Y, et al. Enhanced antibacterial activity of acid treated MgO nanoparticles on Escherichia coli. RSC Advances, 2021, 11(60): 38202. |
| [22] | CHEN Q, GARCIA RP, MUNOZ J, et al. Cellulose nanocrystals-- bioactive glass hybrid coating as bone substitutes by electrophoretic co-deposition: in situ control of mineralization of bioactive glass and enhancement of osteoblastic performance. ACS Applied Materials & Interfaces, 2015, 7(44): 24715. |
| [23] | LIU X, XIE Z, ZHANG C, et al. Bioactive borate glass scaffolds: in vitro and in vivo evaluation for use as a drug delivery system in the treatment of bone infection. Journal Materials Science: Materials in Medcine, 2010, 21(2): 575. |
| [24] |
HOSSEINBABAEI F, RAISSIDEHKORDI B. Electrophoretic deposition of MgO thick films from an acetone suspension. Journal of the European Ceramic Society, 2000, 20(12): 2165.
DOI URL |
| [25] | 董自艳, 戴翚, 马仕洪, 等.紫外-可见分光光度法快速确定细菌菌液的浓度. 中国药品标准, 2014, 15(2): 120. |
| [26] |
KIM D Y, KIM M, KIM H E, et al. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomaterialia, 2009, 5(6): 2196.
DOI URL |
| [27] |
FAN X, FENG B, DI Y, et al. Preparation of bioactive TiO film on porous titanium by micro-arc oxidation. Applied Surface Science, 2012, 258(19): 7584.
DOI URL |
| [28] |
DAGHIGHI S, SJOLLEMA J, VAN DER MEI H C, et al. Infection resistance of degradable versus non-degradable biomaterials: an assessment of the potential mechanisms. Biomaterials, 2013, 34(33): 8013.
DOI PMID |
| [29] |
MUÑIZ DIAZ R, CARDOSO-AVILA P E, PÉREZ TAVARES J A, et al. Two-step triethylamine-based synthesis of MgO nanoparticles and their antibacterial effect against pathogenic bacteria. Nanomaterials, 2021, 11(2): 410.
DOI URL |
| [30] |
TAN J, LIU Z, WANG D, et al. A facile and universal strategy to endow implant materials with antibacterial ability via alkalinity disturbing bacterial respiration. Biomaterials Science, 2020, 8(7): 1815.
DOI URL |
| [31] | LEUNG Y H, NG A M C, XU X, et al. Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small, 2014, 10(6): 1171. |
| [32] | RICKER A, LIU-SNYDER P, WEBSTER T J. The influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cement. International Journal of Nanomedicine, 2008, 3(1): 125. |
| [33] | DEMIREL M. Mechanical properties and cell viability of MgO- reinforced biografts fabricated for biomedical applications. Acta of Bioengineering and Biomechanics, 2018, 20(4): 83. |
| [34] |
JANNING C, WILLBOLD E, VOGT C, et al. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomaterialia, 2010, 6(5): 1861.
DOI PMID |
| [35] | AGARWAL S, CURTIN J, DUFFY B, et al. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications. Materials Science and Engineering: C, 2016, 68: 948. |
| [36] |
ZHU B, WANG L, WU Y, et al. Improving corrosion resistance and biocompatibility of AZ31 magnesium alloy by ultrasonic cold forging and micro-arc oxidation. Journal of Biomaterials Applications, 2022, 36(9): 1664.
DOI PMID |
| [37] |
ZHUANG J, JING Y, WANG Y, et al. Degraded and osteogenic properties of coated magnesium alloy AZ31: an experimental study. Journal of Orthopaedic Surgery and Research, 2016, 11(1): 1.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 何国强, 张恺恒, 王震涛, 包健, 席兆琛, 方振, 王昌昊, 王威, 王鑫, 姜佳沛, 李祥坤, 周迪. Ba(Nd1/2Nb1/2)O3: 一种被低估的K40微波介质陶瓷[J]. 无机材料学报, 2025, 40(6): 639-646. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [5] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [6] | 王智祥, 陈莹, 逄清阳, 李鑫, 王根水. 碳酸锰掺杂氧化镁基陶瓷的烧结行为和介电性能[J]. 无机材料学报, 2025, 40(1): 97-103. |
| [7] | 王月月, 黄佳慧, 孔红星, 李怀珠, 姚晓红. 载银放射状介孔二氧化硅的制备及其在牙科树脂中的应用[J]. 无机材料学报, 2025, 40(1): 77-83. |
| [8] | 吕昕怿, 相恒阳, 曾海波. 长程有序助力钙钛矿QLED高性能化[J]. 无机材料学报, 2025, 40(1): 111-112. |
| [9] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [10] | 肖梓晨, 何世豪, 邱诚远, 邓攀, 张威, 戴维德仁, 缑炎卓, 李金华, 尤俊, 王贤保, 林俍佑. 钙钛矿太阳能电池纳米纤维改性电子传输层研究[J]. 无机材料学报, 2024, 39(7): 828-834. |
| [11] | 张慧, 许志鹏, 朱从潭, 郭学益, 杨英. 大面积有机-无机杂化钙钛矿薄膜及其光伏应用研究进展[J]. 无机材料学报, 2024, 39(5): 457-466. |
| [12] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [13] | 于嫚, 高荣耀, 秦玉军, 艾希成. 上转换发光纳米材料对钙钛矿太阳能电池迟滞效应和离子迁移动力学的影响[J]. 无机材料学报, 2024, 39(4): 359-366. |
| [14] | 王兆阳, 秦鹏, 蒋胤, 冯小波, 杨培志, 黄富强. 三明治结构钌插层二氧化钛光催化四环素降解性能研究[J]. 无机材料学报, 2024, 39(4): 383-389. |
| [15] | 李承瑜, 丁自友, 韩颖超. 锰掺杂纳米羟基磷灰石的体外抗菌-促成骨性能研究[J]. 无机材料学报, 2024, 39(3): 313-320. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||