无机材料学报 ›› 2018, Vol. 33 ›› Issue (9): 931-941.DOI: 10.15541/jim20170585 CSTR: 32189.14.10.15541/jim20170585
张鹏, 张晴, 刘静, 高濂
收稿日期:2017-12-07
修回日期:2018-03-04
出版日期:2018-09-20
网络出版日期:2018-08-14
基金资助:ZHANG Peng, ZHANG Qing, LIU Jing, GAO Lian
Received:2017-12-07
Revised:2018-03-04
Published:2018-09-20
Online:2018-08-14
Supported by:摘要:
甲烷干气重整反应能够实现温室气体CO2和CH4的转化利用, 其反应产物合成气可以通过费托反应进一步生产液态燃料, 该反应在能源与环境领域具有重要意义。寻找合适的催化剂是推动甲烷干气重整工业化的关键。镍基复合结构催化剂因其与贵金属催化剂相媲美的催化活性和低廉的工业成本而受到广泛关注, 但镍基催化剂存在高温下长时间反应后碳沉积和金属组分烧结所导致的失活问题, 严重影响了其工业应用和干气重整化工的发展。本文从镍基复合结构催化剂的成分、结构、制备方法及模拟计算设计等方面出发, 介绍了改进镍基催化剂活性、抗积碳和抗烧结性能的研究进展, 并结合最新的原子催化以及原位表征等研究进展对干气重整研究的发展趋势进行 展望。
中图分类号:
张鹏, 张晴, 刘静, 高濂. 甲烷干气重整镍基复合结构催化剂的研究进展[J]. 无机材料学报, 2018, 33(9): 931-941.
ZHANG Peng, ZHANG Qing, LIU Jing, GAO Lian. Research Progress of Ni-based Composite Catalysts for Methane Dry Reforming[J]. Journal of Inorganic Materials, 2018, 33(9): 931-941.
| Reaction | Reaction equation | △G | ΔH298/(kJ·mol-1) | Limit temperature/K |
|---|---|---|---|---|
| DRM | CH4+CO2→2H2+2CO | 61770-67.32T | 247.3 | 913 |
| RWGS | CO2+H2→CO+H2O | -8545+7.84T | 41.0 | 1093 |
| Boudouard reaction | 2CO→CO2+C | -39810+40.87T | -171.0 | 973 |
| Methane cracking | CH4→C+2H2 | 2190-26.45T | 75.0 | 830 |
表1 甲烷干气重整过程中主要化学反应[12,13]
Table 1 Main chemical reactions during methane dry reforming process[12,13]
| Reaction | Reaction equation | △G | ΔH298/(kJ·mol-1) | Limit temperature/K |
|---|---|---|---|---|
| DRM | CH4+CO2→2H2+2CO | 61770-67.32T | 247.3 | 913 |
| RWGS | CO2+H2→CO+H2O | -8545+7.84T | 41.0 | 1093 |
| Boudouard reaction | 2CO→CO2+C | -39810+40.87T | -171.0 | 973 |
| Methane cracking | CH4→C+2H2 | 2190-26.45T | 75.0 | 830 |
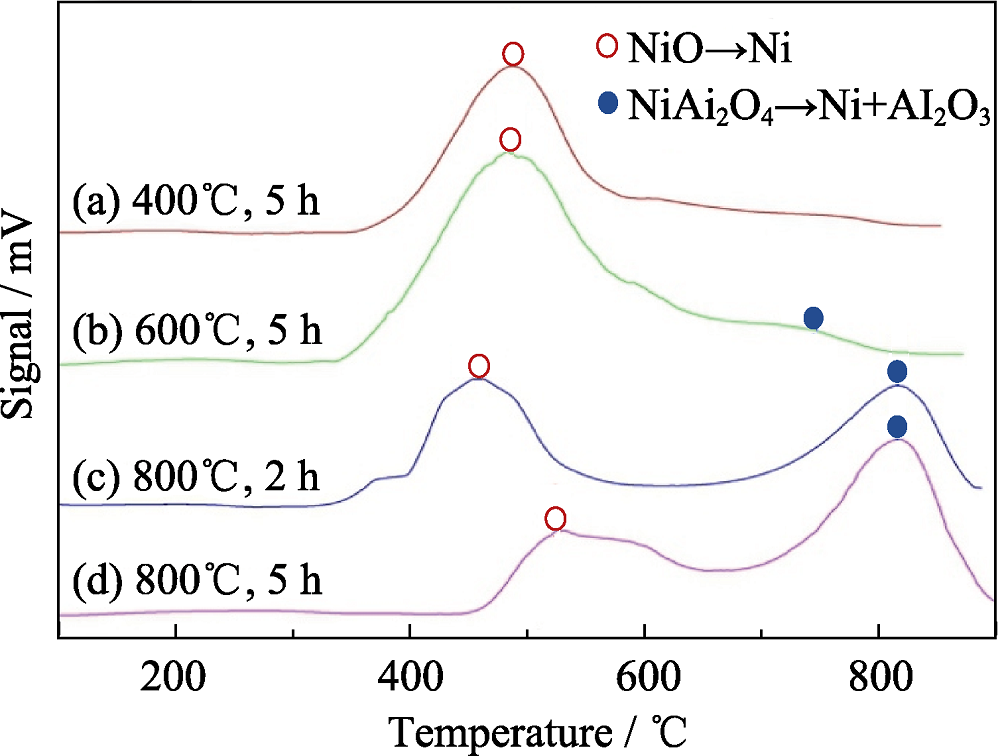
图2 不同煅烧温度和煅烧时间的NiO-γ-Al2O3样品的程序升温还原(TPR)曲线[21]
Fig. 2 Temperature programmed reduction (TPR) curves of NiO-γ-Al2O3 calcined at different temperatures for different durations[21]
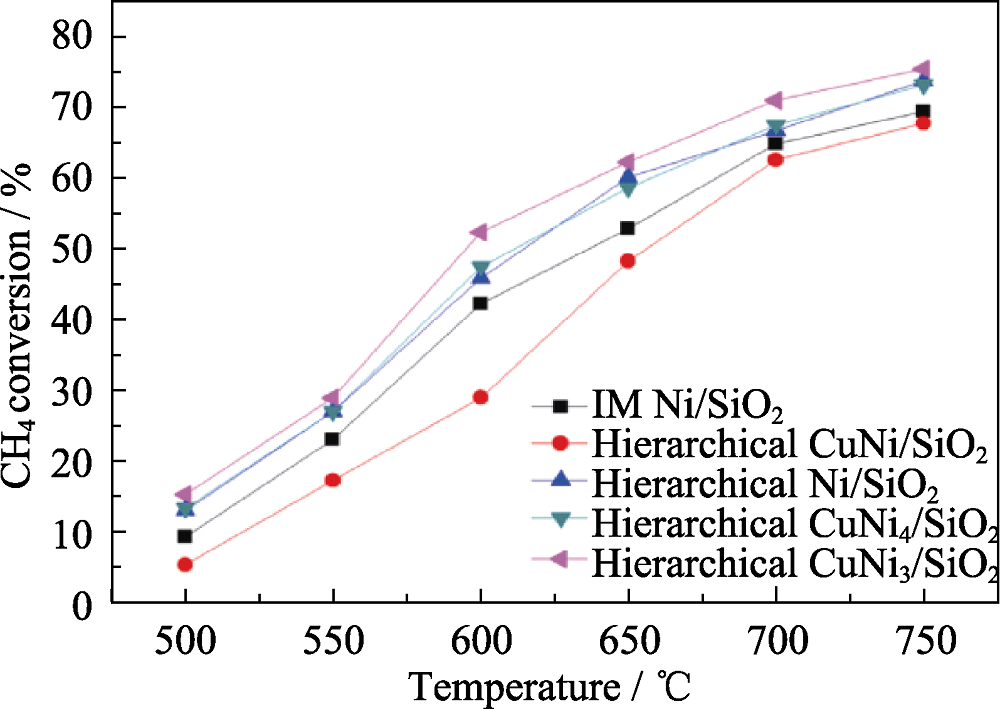
图3 具有不同Cu/Ni比值的Cu-Ni/SiO2催化剂及对照样IM Ni/SiO2在不同温度下用于甲烷干气重整反应的甲烷转化率[22]
Fig. 3 Methane conversion of the methane dry reforming catalysis on the hierarchical Cu-Ni/SiO2 catalysts with various Cu/Ni ratios and the control sample IM Ni/SiO2 at different temperatures[22]
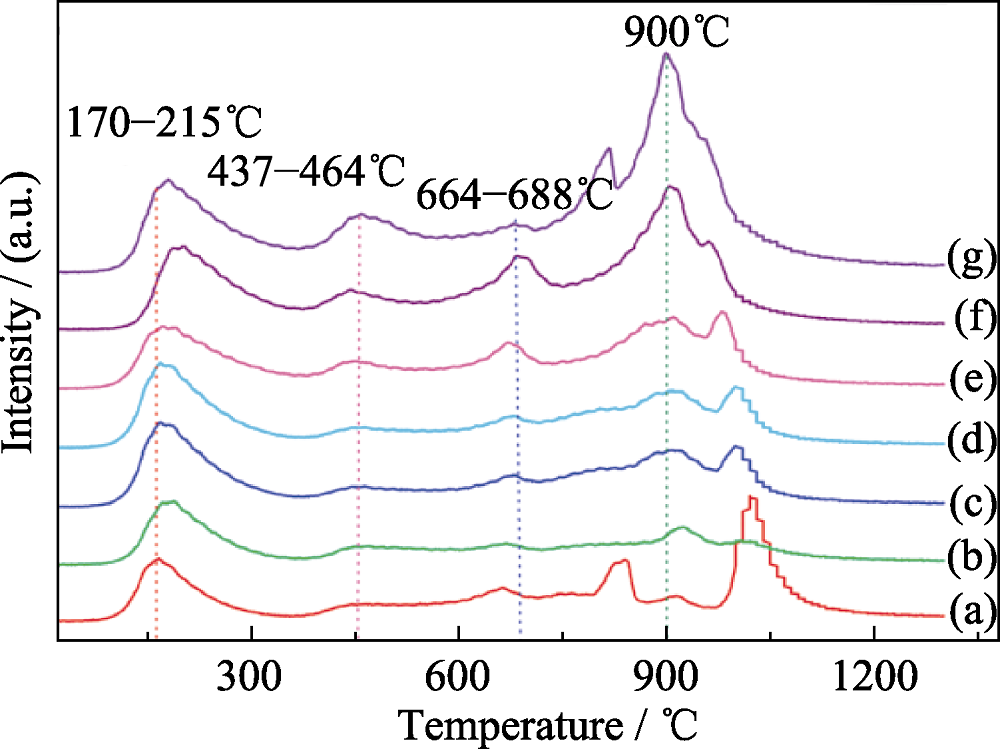
图4 不同Ca含量的M-5NixCa(95-x)Al在600℃煅烧后的CO2-TPD曲线[36]
Fig. 4 CO2-TPD profiles of the M-5NixCa(95-x)Al materials calcined at 600℃ with different Ca contents[36] (a) M-5Ni95Al; (b) M-5Ni1Ca94Al; (c) M-5Ni2Ca93Al; (d) M-5Ni3Ca92Al; (e) M-5Ni5Ca90Al; (f) M-5Ni8Ca87Al; (g) M-5Ni10Ca85Al
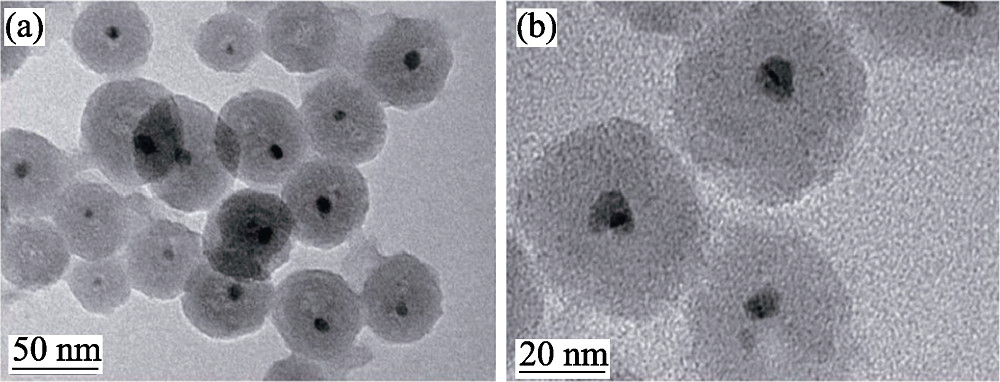
图6 通过标准路线制备的CuNi@SiO2核壳结构催化剂的TEM照片[61]
Fig. 6 TEM images of CuNi@SiO2 core-shell catalysts prepared in the typical synthesis route, a single silica shell contains one core particle[61]
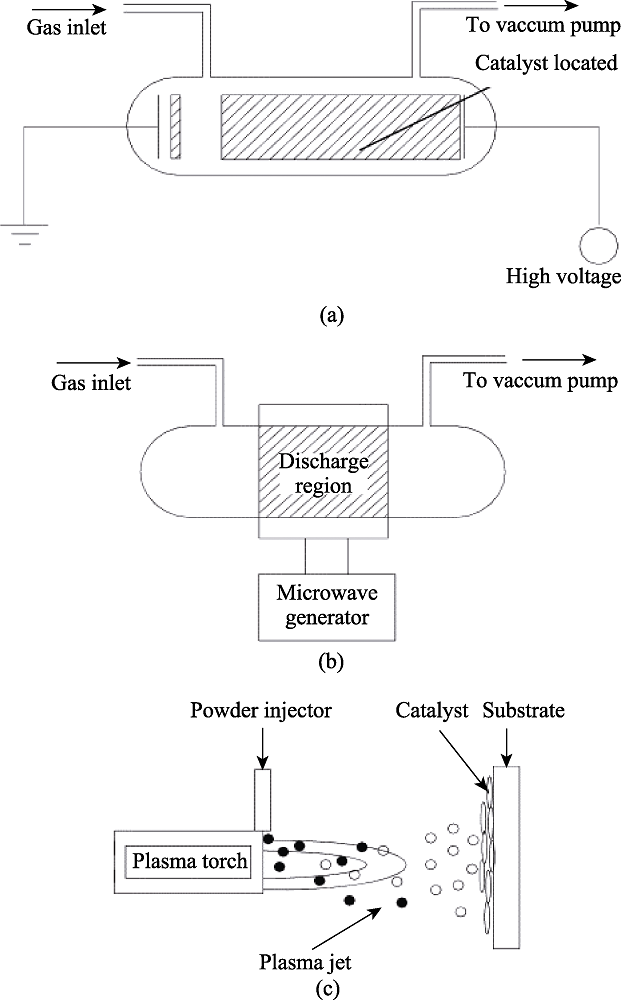
图9 用于制备催化剂的放电电极结构示意图[65]
Fig. 9 Schematically representatives of electrode configurations of discharge phenomena applied for catalyst preparation[65](a) Glow discharge; (b) Microwave plasma; (c) Plasma spraying
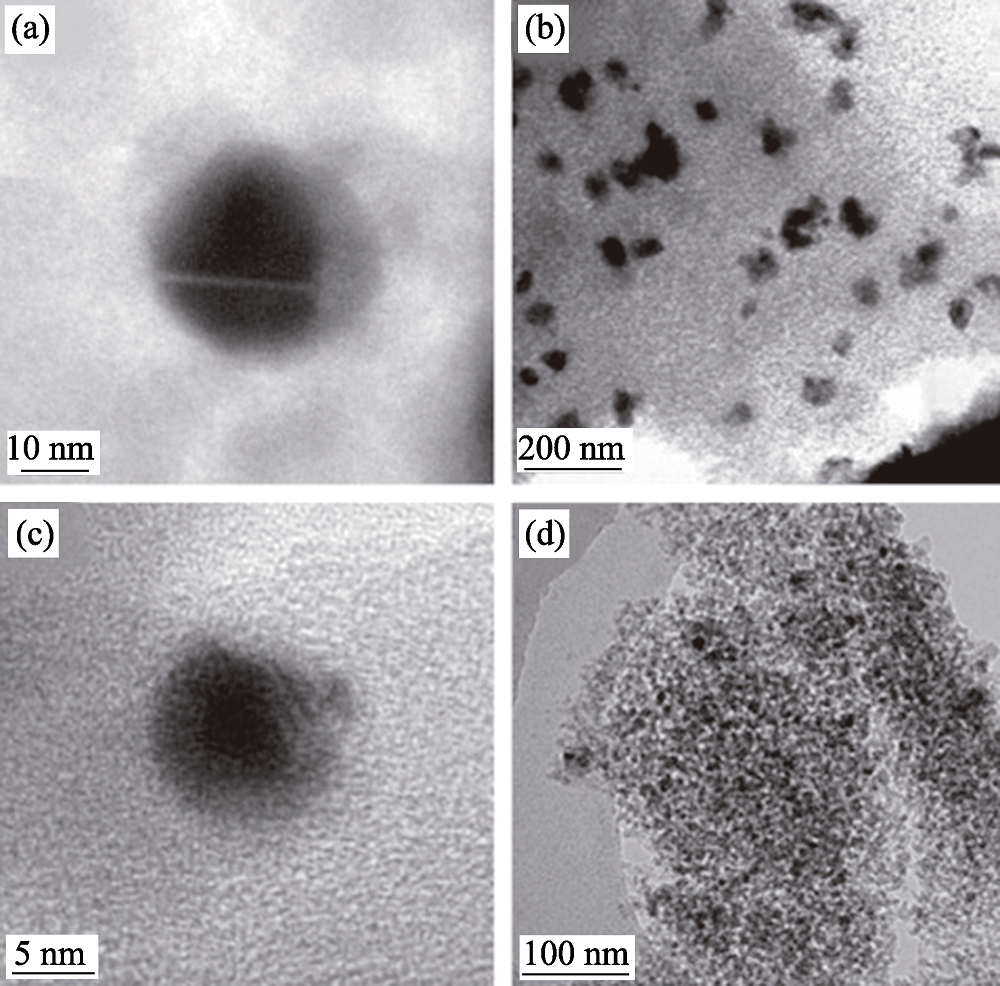
图10 通过两种路线制备的(a)~(b)常规催化剂和(c)~(d)等离子体法制备的新型催化剂的TEM照片[69]
Fig. 10 TEM images of Ni/SiO2 catalyst samples prepared by two methods (a, b) the conventional catalyst and (c, d) the novel catalyst prepared by plasma jet[69]
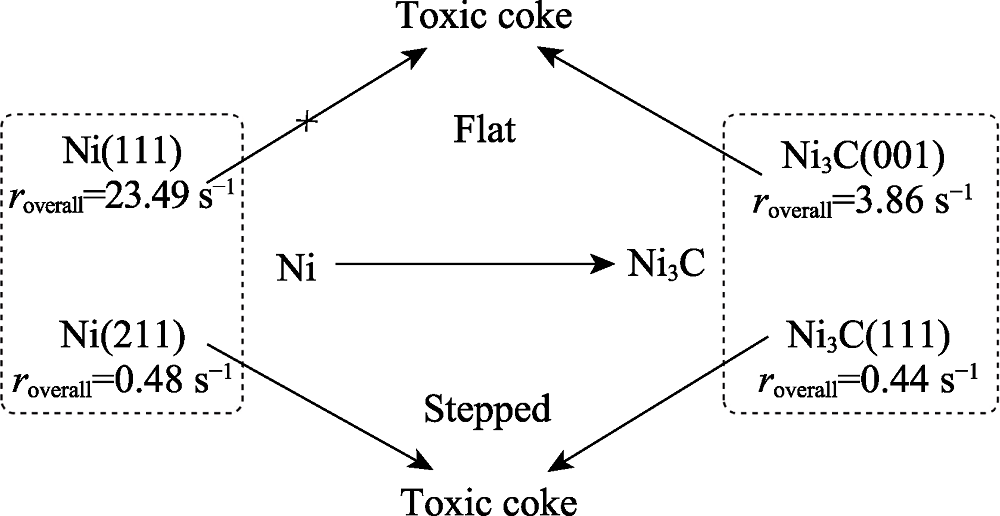
图11 相变及积碳造成的失活示意图[71]
Fig. 11 Deactivation scheme to show schematically the phase transformation and coke formation[71]Ni(111) and Ni(211) stand for the clean nickel metal flat and stepped surfaces respectively, and Ni3C(001) and Ni3C(111) are the flat and stepped nickel carbide. Flat surfaces are marked in blue, while stepped surfaces are in red. Toxic coke is the more stable and high toxicity carbon atom which will result in the deactivation of the nickel catalyst. The corresponding overall reaction rates are marked below the different structures. The arrow with X means this step is not likely to occur, while arrow without X means this step could happen
| [1] | TOLLEFSON J.World looks ahead post-Copenhagen.Nature, 2009, 462(7276): 966-967. |
| [2] | TOLLEFSON J.Copenhagen: the scientists' view.Nature, 2009, 462(7274): 714. |
| [3] | WANG W, WANG S P, MA X B,et al. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev., 2011, 40(7): 3703-3727. |
| [4] | TOLLEFSON J.World’s carbon emissions set to spike by 2% in 2017.Nature News, 2017, 551(7680): 283. |
| [5] | SONG C S.Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing.Catal. Today, 2006, 115(1-4): 2-32. |
| [6] | MELIKOGLU M.Shale gas: analysis of its role in the global energy market.Renew Sust. Energ. Rev., 2014, 37: 460-468. |
| [7] | BERNARD S, HORSFIELD B.Thermal maturation of gas shale systems.Annu. Rev. Earth Planet. Sci., 2014, 42(1): 635-651. |
| [8] | WANG N, YU X, SHEN K,et al. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy, 2013, 38(23): 9718-9731. |
| [9] | SIBUDJING K, YASOTHA K, JUN N,et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem, 2015, 8(21): 3556-3575. |
| [10] | ASSABUMRUNGRAT S, CHAROENSERI S, LAOSIRIPOJANA N,et al. Effect of oxygen addition on catalytic performance of Ni/SiO·MgO toward carbon dioxide reforming of methane under periodic operation. Int. J. Hydrogen Energy, 2009, 34(15): 6211-6220. |
| [11] | KIM H Y, PARK J N, HENKELMAN G,et al. Design of a highly nanodispersed Pd-MgO/SiO2 composite catalyst with multifunctional activity for CH4 reforming. ChemSusChem, 2012, 5(8): 1474-1481. |
| [12] | FAN M S, ABDULLAH A Z, BHATIA S.Catalytic technology for carbon dioxide reforming of methane to synthesis gas.ChemCatChem, 2009, 1(2): 192-208. |
| [13] | WANG S, LU G Q, MILLAR G J.Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art. Energy & Fuels, 1996, 10(4): 896-904. |
| [14] | ZHANG J, WANG H, DALAI A K.Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal., 2007, 249(2): 300-310. |
| [15] | TOPALIDIS A, PETRAKIS D, LADAVOS A,et al. A kinetic study of methane and carbon dioxide interconversion over 0.5% Pt/SrTiO3 catalysts. Catal. Today, 2007, 127(1): 238-245. |
| [16] | PAKHARE D, SPIVEY J.A review of dry (CO2) reforming of methane over noble metal catalysts.Chem. Soc. Rev., 2014, 43(22): 7813-7837. |
| [17] | BRADFORD M C J, VANNICE M A. CO2 reforming of CH4 over supported Pt catalysts.J. Catal., 1998, 173(1): 157-171. |
| [18] | BIAN Z, SURYAWINATA I Y, KAWI S.Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane.Appl. Catal., B, 2016, 195: 1-8. |
| [19] | TSYGANOK A I, TSUNODA T, HAMAKAWA S,et al. Dry reforming of methane over catalysts derived from nickel-containing Mg-Al layered double hydroxides. J. Catal., 2003, 213(2): 191-203. |
| [20] | LIU C J, YE J, JIANG J,et al. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemSusChem, 2015, 3(3): 529-541. |
| [21] | ZHANG Q, WU T, ZHANG P,et al. Facile synthesis of hollow hierarchical Ni/g-Al2O3 nanocomposites for methane dry reforming catalysis. RSC Adv., 2014, 4(93): 51184-51193. |
| [22] | WU T, ZHANG Q, CAI W,et al. Phyllosilicate evolved hierarchical Ni- and Cu-Ni/SiO2 nanocomposites for methane dry reforming catalysis. Appl. Catal. A, 2015, 503: 94-102. |
| [23] | DAZA C E, GALLEGO J, MONDRAG N F,et al. High stability of Ce-promoted Ni/Mg-Al catalysts derived from hydrotalcites in dry reforming of methane. Fuel, 2010, 89(3): 592-603. |
| [24] | FERREIRA-APARICIO P, FERNANDEZ-GARCIA M, GUERRERO-RUIZ A,et al. Evaluation of the role of the metal-support interfacial centers in the dry reforming of methane on alumina- supported rhodium catalysts. J. Catal., 2000, 190(2): 296-308. |
| [25] | CHETTAPONGSAPHAN C, CHAROJROCHKUL S, ASSABUMRUNGRAT S,et al. Catalytic H2O and CO2 reforming of CH4 over perovskite-based La0.8Sr0.2Cr0.9Ni0.1O3: effects of pre-treatment and co-reactant/CH4 on its reforming characteristics. Appl. Catal., 2010, 386(1/2): 194-200. |
| [26] | GALLEGO G S, BATIOT-DUPEYRAT C, BARRAULT J,et al. Dry reforming of methane over LaNi1- yByO3±δ(B= Mg, Co) perovskites used as catalyst precursor. Appl. Catal. A, 2008, 334(1/2): 251-258. |
| [27] | GARC A-DI GUEZ M, PIETA I, HERRERA M,et al. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3. Catal. Today, 2010, 149(3/4): 380-387. |
| [28] | LUCR DIO A F, ASSAF J M, ASSAF E M. Methane conversion reactions on Ni catalysts promoted with Rh: influence of support.Appl. Catal. A, 2011, 400(1/2): 156-165. |
| [29] | HAN J W, KIM C, PARK J S,et al. Highly coke-resistant Ni nanoparticle catalysts with minimal sintering in dry reforming of methane. ChemSusChem, 2014, 7(2): 451-456. |
| [30] | ALIEH, KHALESI, HAMID, et al. Effects of lanthanum substitution by strontium and calcium in La-Ni-Al perovskite oxides in dry reforming of methane. Chin. J. Catal., 2008, 29(10): 960-968. |
| [31] | LI X, LI D, TIAN H,et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B, 2017, 202: 683-694. |
| [32] | LUNA A E C, IRIARTE M E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst.Appl. Catal. A, 2008, 343(1): 10-15. |
| [33] | XU Z, ZHEN M, BI Y,et al. Catalytic properties of Ni modified hexaaluminates LaNiyAl12-yO19-δ for CO2 reforming of methane to synthesis gas. Appl. Catal., A, 2000, 198(1): 267-723. |
| [34] | HU Y H.Solid-solution catalysts for CO2 reforming of methane.Catal. Today, 2009, 148(3): 206-211. |
| [35] | JAFARBEGLOO M, TARLANI A, MESBAH A W,et al. NiO-MgO Solid solution prepared by Sol-Gel method as precursor for Ni/MgO methane dry reforming catalyst: effect of calcination temperature on catalytic performance. Catal. Lett., 2016, 146(1): 238-248. |
| [36] | XU L, SONG H, CHOU L.One-pot synthesis of ordered mesoporous NiO-CaO-Al2O3 composite oxides for catalyzing CO2 reforming of CH4.ACS Catalysis, 2012, 2(7): 1331-1342. |
| [37] | ZUBENKO D, SINGH S, ROSEN B A.Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming.Appl. Catal. B, 2017, 209: 711-719. |
| [38] | LI Y, ZHANG Q, LIU J,et al. Corona shaped SiO2 supported Ni nanoparticles for methane dry reforming catalysis. J. Chin. Ceram. Soc., 2015, 43(7): 911-918. |
| [39] | BIAN Z, DAS S, WAI M H,et al. A review on bimetallic Ni-based catalysts for CO2 reforming of methane. ChemPhysChem A, 2017, 18(22): 3117-3134 |
| [40] | TU W, GHOUSSOUB M, SINGH C V,et al. Consequences of surface oxophilicity of Ni, Ni-Co, and Co clusters on methane activation. J. Am. Chem. Soc., 2017, 139(20): 6928-6945. |
| [41] | CHEN Y G, YAMAZAKI O, TOMISHIGE K,et al. Noble metal promoted Ni0.03Mg0.97O solid solution catalysts for the reforming of CH4 with CO2. Catal. Lett., 1996, 39(1/2): 91-95. |
| [42] | WU H, PANTALEO G, PAROLA V L, ,et al. Bi-. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MOx (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B, 2014, 156-157(2): 350-361. |
| [43] | HORIUCHI T, SAKUMA K, FUKUI T,et al. Suppression of carbon deposition in the CO2 -reforming of CH4 by adding basic metal oxides to a Ni/Al2O3 catalyst. Appl. Catal. A, 1996, 144(1/2): 111-120. |
| [44] | OSAKI T, MORI T.Role of potassium in carbon-free CO2 reforming of methane on K-promoted Ni/Al2O3 catalysts.J. Catal., 2001, 204(1): 89-97. |
| [45] | FRUSTERI F, SPADARO L, ARENA F,et al. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni/MgO catalysts during the dry reforming of methane. Carbon, 2002, 40(7): 1063-1070. |
| [46] | DIAS J A C, ASSAF J M. Influence of calcium content in Ni/CaO/γ-Al2O3 catalysts for CO2-reforming of methane. Catal. Today, 2003, 85(1): 59-68. |
| [47] | KOO K Y, ROH H S, YU T S,et al. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A, 2008, 340(2): 183-190. |
| [48] | KATHIRASER Y, THITSARTARN W, SUTTHIUMPORN K,et al. Inverse NiAl2O4 on LaAlO3-Al2O3: unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C, 2013, 117(16): 8120-8130. |
| [49] | SUTTHIUMPORN K, KAWI S.Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: role of surface oxygen species on H2 production and carbon suppression.Int. J. Hydrogen Energy, 2011, 36(22): 14435-14446. |
| [50] | STAGG-WILLIAMS S M, FENDLEY G, RESASCO D E,et al. CO2 reforming of CH4 over Pt/ZrO2 catalysts promoted with La and Ce oxides. J. Catal., 2000, 194(2): 240-249. |
| [51] | VALENTINI A, PROBST L F D, LISBOA-FILHO P N, et al. Role of vanadium in Ni:Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. A, 2003, 255(2): 211-220. |
| [52] | PAN Y, KUAI P, LIU Y,et al. Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Ni catalyst. Energy Environ. Sci., 2010, 3(9): 1322-1325. |
| [53] | HOU Z, YOKOTA O, TANAKA T,et al. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A, 2003, 253(2): 381-387. |
| [54] | GUO J, LOU H, ZHAO H,et al. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A, 2004, 273(1): 75-82. |
| [55] | MARGOSSIAN T, LARMIER K, KIM S M,et al. Molecularly- tailored nickel precursor and support yield a stable methane dry reforming catalyst with superior metal utilization. J. Am. Chem. Soc., 2017, 139(20): 6919-6927. |
| [56] | LIU S, BAI S Q, ZHENG Y,et al. Composite metal-oxide nanocatalysts. ChemCatChem, 2012, 4(10): 1462-1484. |
| [57] | ZHANG J, LI F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B, 2015, s176-177: 513-521. |
| [58] | KIM D H, KIM S Y, HAN S W,et al. The catalytic stability of TiO2-shell/Ni-core catalysts for CO2 reforming of CH4. Appl. Catal. A, 2015, 495: 184-191. |
| [59] | BAKTASH E, LITTLEWOOD P, SCHOM CKER R,et al. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B, 2015, 179: 122-127. |
| [60] | LIM Z Y, WU C, WANG W G,et al. Porosity effect on ZrO2 hollow shells and hydrothermal stability for catalytic steam reforming of methane. Journal of Materials Chemistry A, 2015, 4(1): 153-159. |
| [61] | WU T, CAI W, ZHANG P,et al. Cu-Ni@SiO2 alloy nanocomposites for methane dry reforming catalysis. RSC Adv., 2013, 3(46): 23976-23979. |
| [62] | DU X, ZHANG D, SHI L,et al. Coke- and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale, 2013, 5(7): 2659-2663. |
| [63] | LI W, ZHAO Z, JIAO Y.Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets.Int. J. Hydrogen Energy, 2016, 41(40): 17907-17921. |
| [64] | ZHU X, HUO P, ZHANG Y P,et al. Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl. Catal. B, 2008, 81(1/2): 132-140. |
| [65] | LIU C J, VISSOKOV G P, JANG W L.Catalyst preparation using plasma technologies.Catal. Today, 2002, 72(3/4): 173-184. |
| [66] | LIU C J.Plasma application for more environmentally friendly catalyst preparation. Pure Appl. Chem., 2006, 78(6): 1227-1238. |
| [67] | HONG J, CHU W, CHERNAVSKII P A,et al. Cobalt species and cobalt-support interaction in glow discharge plasma-assisted Fischer- Tropsch catalysts. J. Catal., 2010, 273(1): 9-17. |
| [68] | CHENG D G, ZHU X, BEN Y,et al. Carbon dioxide reforming of methane over Ni/Al2O3 treated with glow discharge plasma. Catal. Today, 2006, 115(1-4): 205-210. |
| [69] | LIU G, LI Y, CHU W,et al. Plasma-assisted preparation of Ni/SiO2 catalyst using atmospheric high frequency cold plasma jet. Catal. Commun., 2008, 9(6): 1087-1091. |
| [70] | WANG S G, CAO D B, LI Y W,et al. CO2 reforming of CH4 on Ni (111): a density functional theory calculation. J. Phys. Chem. B, 2006, 110(20): 9976-9983. |
| [71] | WANG Z, CAO X M, ZHU J,et al. Activity and coke formation of nickel and nickel carbide in dry reforming: a deactivation scheme from density functional theory. J. Catal., 2014, 311(3): 469-480. |
| [72] | AW M S, ZORKO M, DJINOVIĆ P,et al. Insights into durable NiCo catalysts on β-SiC/CeZrO2 and γ-Al2O3/CeZrO2 advanced supports prepared from facile methods for CH4-CO2 dry reforming. Appl. Catal. B, 2015, 164: 100-112. |
| [73] | OEMAR U, HIDAJAT K, KAWI S.Role of catalyst support over PdO-NiO catalysts on catalyst activity and stability for oxy-CO2 reforming of methane.Appl. Catal. A, 2011, 402(1/2): 176-187. |
| [74] | NI J, CHEN L, LIN J,et al. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy, 2012, 1(5): 674-686. |
| [75] | HAN J, ZHAN Y, STREET J,et al. Natural gas reforming of carbon dioxide for syngas over Ni-Ce-Al catalysts. Int. J. Hydrogen Energy, 2017, 42(29): 18364-18374. |
| [76] | CHEN Q, ZHANG J, PAN B,et al. Temperature-dependent anti-coking behaviors of highly stable Ni-CaO-ZrO2 nanocomposite catalysts for CO2 reforming of methane. Chem. Eng. J., 2017, 320: 63-73. |
| [77] | THEOFANIDIS S A, GALVITA V V, SABBE M,et al. Controlling the stability of a Fe-Ni reforming catalyst: structural organization of the active components. Appl. Catal. B, 2017, 209: 405-416. |
| [1] | 王海龙, 王扬, 王向伟, 张宏志. 热裂法切割玻璃等硬脆材料关键技术研究进展[J]. 无机材料学报, 2018, 33(9): 923-930. |
| [2] | 李江, 戴佳卫, 潘裕柏. 磁光透明陶瓷的研究进展[J]. 无机材料学报, 2018, 33(1): 1-8. |
| [3] | 程 亮, 罗凌虹, 石纪军, 孙良良, 徐 序, 吴也凡, 胡佳幸. Ni/YSZ阳极浸渍La2O3对SOFC电池抗积碳的影响[J]. 无机材料学报, 2017, 32(3): 241-246. |
| [4] | 郑艳彬, 姜志刚, 朱品文. 洋葱碳的制备与应用研究进展[J]. 无机材料学报, 2015, 30(8): 793-801. |
| [5] | 刘 岗, 严 岩. 冷冻干燥法制备多孔陶瓷研究进展[J]. 无机材料学报, 2014, 29(6): 571-583. |
| [6] | 杨培志,刘黎明,张小文,莫镜辉. 长波红外光学材料的研究进展[J]. 无机材料学报, 2008, 23(4): 641-646. |
| [7] | 江奇,瞿美臻,张伯兰,于作龙. 电化学超级电容器电极材料的研究进展[J]. 无机材料学报, 2002, 17(4): 649-656. |
| [8] | 李冬云,乔冠军,金志浩. 层状复合陶瓷材料的研究进展[J]. 无机材料学报, 2002, 17(1): 10-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||