无机材料学报 ›› 2021, Vol. 36 ›› Issue (4): 372-378.DOI: 10.15541/jim20200374 CSTR: 32189.14.10.15541/jim20200374
张丰年( ), 郭猛, 苗洋(
), 郭猛, 苗洋( ), 高峰, 成楚飞, 程富豪, 刘宇峰
), 高峰, 成楚飞, 程富豪, 刘宇峰
收稿日期:2020-07-06
修回日期:2020-08-28
出版日期:2021-04-20
网络出版日期:2021-04-19
通讯作者:
苗洋, 副教授. E-mail: miaoyang@tyut.edu.cn
作者简介:张丰年(1998-), 男, 硕士研究生. E-mail: zhangfn1998@163.com
基金资助:
ZHANG Fengnian( ), GUO Meng, MIAO Yang(
), GUO Meng, MIAO Yang( ), GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng
), GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng
Received:2020-07-06
Revised:2020-08-28
Published:2021-04-20
Online:2021-04-19
Contact:
MIAO Yang, associate professor. E-mail: miaoyang@tyut.edu.cn
About author:ZHANG Fengnian(1998-), male, Master candidate. E-mail: zhangfn1998@163.com
Supported by:摘要:
近年来, 不同体系的高熵陶瓷迅猛发展, 但萤石结构高熵氧化物仍处于研发初期。本研究采用机械球磨和常压烧结的方法合成一种新型高熵萤石氧化物, 利用XRD, SEM, TG-DSC和可视化形变分析仪研究了陶瓷的物相转变、表面形貌以及烧结行为。研究结果表明, (Zr1/7Hf1/7Ce1/7Y2/7La2/7)O2-δ是一种非等摩尔的“高熵”陶瓷, 其内部各元素分布均匀。湿法球磨和干法球磨制备的粉末结构和粒度均有所不同, 单相转变温度为1600和1300 ℃, 圆盘状坯体在1600 ℃保温1 h, 尺寸收缩率约为8.5%和17.8%, 致密度分别为82.25%和93.23%, 说明球磨工艺是影响高熵陶瓷烧结的重要因素。此外, 常压烧结制备(Zr1/7Hf1/7Ce1/7Y2/7La2/7)O2-δ时, 在1300~1600 ℃应适当减缓升温速率并延长保温时间, 避免生坯收缩开裂, 并提高陶瓷致密度。
中图分类号:
张丰年, 郭猛, 苗洋, 高峰, 成楚飞, 程富豪, 刘宇峰. 高熵陶瓷(Zr1/7Hf1/7Ce1/7Y2/7La2/7)O2-δ的制备及烧结行为[J]. 无机材料学报, 2021, 36(4): 372-378.
ZHANG Fengnian, GUO Meng, MIAO Yang, GAO Feng, CHENG Chufei, CHENG Fuhao, LIU Yufeng. Preparation and Sintering Behavior of High Entropy Ceramic (Zr1/7Hf1/7Ce1/7Y2/7La2/7)O2-δ[J]. Journal of Inorganic Materials, 2021, 36(4): 372-378.
| Oxides | Crystal structure | Space group (number) | CN | rc /nm |
|---|---|---|---|---|
| ZrO2 | Cubic | Fm-3m (225) | 8 | 0.084 |
| HfO2 | Monoclinic | P21/c (14) | 8 | 0.083 |
| CeO2 | Fluorite | Fm-3m (225) | 8 | 0.097 |
| Y2O3 | Bixbyite | Ia-3 (206) | 6 | 0.090 |
| La2O3 | Trigonal | P-3m (164) | 6 | 0.1032 |
表1 氧化物的晶体结构、空间群及对应阳离子配位数和半径[42]
Table 1 Crystal structures, space groups (number), cation coordination numbers (CN) and corresponding cationic radii (rc) of selected oxides[42]
| Oxides | Crystal structure | Space group (number) | CN | rc /nm |
|---|---|---|---|---|
| ZrO2 | Cubic | Fm-3m (225) | 8 | 0.084 |
| HfO2 | Monoclinic | P21/c (14) | 8 | 0.083 |
| CeO2 | Fluorite | Fm-3m (225) | 8 | 0.097 |
| Y2O3 | Bixbyite | Ia-3 (206) | 6 | 0.090 |
| La2O3 | Trigonal | P-3m (164) | 6 | 0.1032 |
| Sample | Process | BPR | Speed /(r·min-1) | Time /h | Disperser | Desiccation | Energy |
|---|---|---|---|---|---|---|---|
| A | Wet milling | 6 : 1 | 250 | 6 | Ethanol | 60 ℃ /24 h | Low |
| B | Dry milling | 10 : 1 | 400 | 40 | - | - | High |
表2 制备生料的工艺、球料比、转速、时间及其他参数
Table 2 Process, ball-to-powder-ratio (BPR), speed, time and others for raw material preparation
| Sample | Process | BPR | Speed /(r·min-1) | Time /h | Disperser | Desiccation | Energy |
|---|---|---|---|---|---|---|---|
| A | Wet milling | 6 : 1 | 250 | 6 | Ethanol | 60 ℃ /24 h | Low |
| B | Dry milling | 10 : 1 | 400 | 40 | - | - | High |
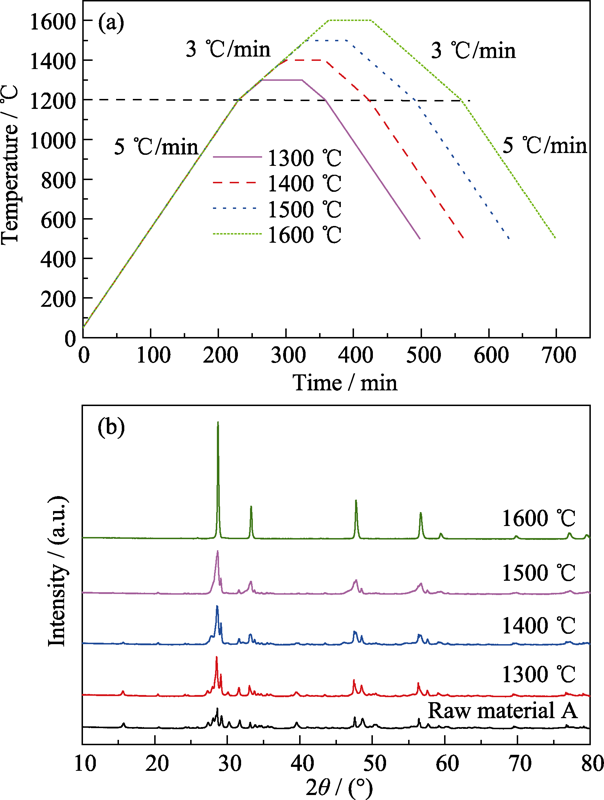
图1 (a)生料的煅烧工艺和(b)不同温度煅烧生料A的XRD图谱
Fig. 1 (a) Calcining process of raw materials and (b) XRD patterns of raw material A calcined at different temperatures
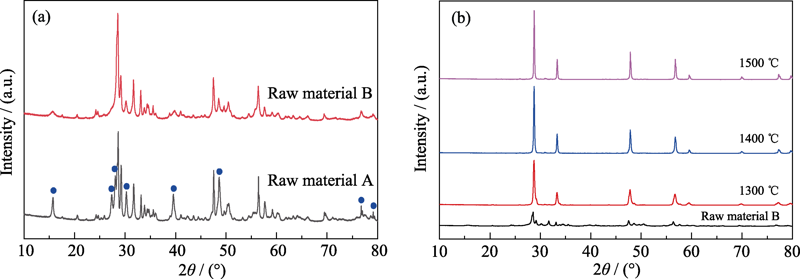
图7 (a) 生料A和B的XRD图谱对比, (b)不同温度下煅烧生料B的XRD图谱
Fig. 7 (a) Comparation of XRD patterns from raw material A and B; (b) XRD patterns of raw material B sintered at different temperatures
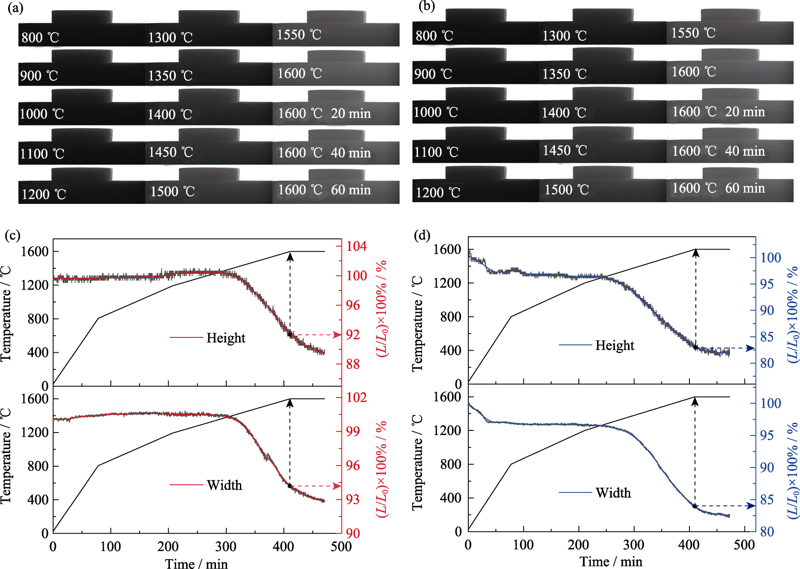
图8 不同坯体在不同温度下烧结的光学照片以及坯体随时间变化的温度/线性收缩
Fig. 8 Digital camera photographs of different pellets sintered at different temperatures and temper ature/linear shrinkage of different pellets as a function of time (a,c) Pellet A; (b,d) Pellet B
| [1] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Materialia, 2017,122:448-511. |
| [2] | ZHU J M, FU H M, ZHANG F, et al. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Materials Science and Engineering: A, 2010,527(27):7210-7214. |
| [3] | SZKLARZ Z, LEKKI J, BOBROWSKI P, et al. The effect of SiC nanoparticles addition on the electrochemical response of mechanically alloyed CoCrFeMnNi high entropy alloy. Materials Chemistry and Physics, 2018,215:385-392. |
| [4] | TSAI M, WANG C W, TSAI C W, et al. Thermal stability and performance of NbSiTaTiZr high-entropy alloy barrier for copper metallization. Journal of The Electrochemical Society, 2011,158(11):H1161-H1165. |
| [5] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nature Communications, 2015,6(1):8485. |
| [6] | WEI X F, LIU J X, LI F, et al. High entropy carbide ceramics from different starting materials. Journal of the European Ceramic Society, 2019,39(10):2989-2994. |
| [7] | JIN T, SANG X H, UNOCIC R R, et al. Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy. Advanced Materials, 2018,30(23):1707512. |
| [8] | LIU J X, SHEN X Q, WU Y, et al. Mechanical properties of hot-pressed high-entropy diboride-based ceramics. Journal of Advanced Ceramics, 2020,9(4):503-510. |
| [9] | QIN Y, LIU J X, LI F, et al. A high entropy silicide by reactive spark plasma sintering. Journal of Advanced Ceramics, 2019,8(1):148-152. |
| [10] |
CHEN X Q, WU Y Q. High-entropy transparent fluoride laser ceramics. Journal of the American Ceramic Society, 2020,103(2):750-756.
DOI URL |
| [11] |
ZHANG R Z, GUCCI F, ZHU H Y, et al. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorganic Chemistry, 2018,57(20):13027-13033.
DOI URL PMID |
| [12] | DJENADIC R, SARKAR A, CLEMENS O, et al. Multicomponent equiatomic rare earth oxides. Materials Research Letters, 2017,5(2):102-109. |
| [13] | MAO A Q, XIANG H Z, ZHANG Z G, et al. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder. Journal of Magnetism and Magnetic Materials, 2019,484:245-252. |
| [14] | XING Q W, XIA S Q, YAN X H, et al. Mechanical properties and thermal stability of (NbTiAlSiZr)Nx high-entropy ceramic films at high temperatures. Journal of Materials Research, 2018,33(19):3347-3354. |
| [15] | CHEN L, WANG K, SU W T, et al. Research progress of transition metal non-oxide high-entropy ceramics. Journal of Inorganic Materials, 2020,35(7):748-758. |
| [16] | CHEN H, QIU N, WU B Z, et al. Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties. RSC Advances, 2019,9(50):28908-28915. |
| [17] | BÉRARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Physica Status Solidi, 2016,10(4):328-333. |
| [18] | ZHANG J J, YAN J Q, CALDER S, et al. Long-range antiferromagnetic order in a rocksalt high entropy oxide. Chemistry of Materials, 2019,31(10):3705-3711. |
| [19] | BéRARDAN D, FRANGER S, MEENA A K, et al. Room temperature lithium superionic conductivity in high entropy oxides. Journal of Materials Chemistry A, 2016,4(24):9536-9541. |
| [20] | CHEN H, FU J, ZHANG P F, et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. Journal of Materials Chemistry A, 2018,6(24):11129-11133. |
| [21] | CHEN H, LIN W W, ZHANG Z H, et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: dispersion of catalysts via entropy maximization. ACS Materials Letters, 2019,1(1):83-88. |
| [22] | SARKAR A, DJENADIC R, WANG D, et al. Rare earth and transition metal based entropy stabilised perovskite type oxides. Journal of the European Ceramic Society, 2018,38(5):2318-2327. |
| [23] | PU Y P, ZHANG Q W, LI R, et al. Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic. Applied Physics Letters, 2019,115(22):223901. |
| [24] | LIU J, REN K, MA C Y, et al. Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic. Ceramics International, 2020,46(12):20576-20581. |
| [25] | EDALATI P, WANG Q, RAZAVI-KHOSROSHAHI H, et al. Photocatalytic hydrogen evolution on a high-entropy oxide. Journal of Materials Chemistry A, 2020,8(7):3814-3821. |
| [26] |
WANG T, CHEN H, YANG Z, et al. High-entropy perovskite fluorides: a new platform for oxygen evolution catalysis. Journal of the American Chemical Society, 2020,142(10):4550-4554.
URL PMID |
| [27] | FRACCHIA M, MANZOLI M, ANSELMI-TAMBURINI U, et al. A new eight-cation inverse high entropy spinel with large configurational entropy in both tetrahedral and octahedral sites: Synthesis and cation distribution by X-ray absorption spectroscopy. Scripta Materialia, 2020,188:26-31. |
| [28] | WANG D, JIANG S D, DUAN C Q, et al. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. Journal of Alloys and Compounds, 2020,844:156158. |
| [29] | MAO A Q, QUAN F, XIANG H Z, et al. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder. Journal of Molecular Structure, 2019,1194:11-18. |
| [30] | WANG J B, STENZEL D, AZMI R, et al. Spinel to rock-salt transformation in high entropy oxides with Li incorporation. Electrochem, 2020,1(1):60-74. |
| [31] | LI F, ZHOU L, LIU J X, et al. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. Journal of Advanced Ceramics, 2019,8(4):576-582. |
| [32] | CHEN H, ZHAO Z F, XIANG H M, et al. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: a novel high temperature stable thermal barrier material. Journal of Materials Science & Technology, 2020,48:57-62. |
| [33] | ZHAO Z F, CHEN H, XIANG H M, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. Journal of Materials Science & Technology, 2019,35(12):2892-2896. |
| [34] | CHEN H, XIANG H M, DAI F Z, et al. High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion. Journal of Materials Science & Technology, 2020,36:134-139. |
| [35] | VINNIK D A, TROFIMOV E A, ZHIVULIN V E, et al. The new extremely substituted high entropy (Ba,Sr,Ca,La)Fe6-x (Al,Ti,Cr,Ga,In,Cu,W)xO19 microcrystals with magnetoplumbite structure. Ceramics International, 2020,46(7):9656-9660. |
| [36] |
VINNIK D A, ZHIVULIN V E, TROFIMOV E A, et al. Extremely polysubstituted magnetic material based on magnetoplumbite with a hexagonal structure: synthesis, structure, properties, prospects. Nanomaterials (Basel), 2019,9(4):559.
DOI URL |
| [37] | SKINNER S J, KILNER J A. Oxygen ion conductors. Materials Today, 2003,6(3):30-37. |
| [38] |
SACHKOV V I, NEFEDOV R A, AMELICHKIN I V. High entropy oxide systems based on rare earth elements. IOP Conference Series: Materials Science and Engineering, 2019,597:012005.
DOI URL |
| [39] | PIANASSOLA M, LOVEDAY M, MCMURRAY J W, et al. Solid-state synthesis of multicomponent equiatomic rare-earth oxides. Journal of the American Ceramic Society, 2020,103(4):2908-2918. |
| [40] |
SARKAR A, LOHO C, VELASCO L, et al. Multicomponent equiatomic rare earth oxides with a narrow band gap and associated praseodymium multivalency. Dalton Trans., 2017,46(36):12167-12176.
DOI URL PMID |
| [41] | GILD J, SAMIEE M, BRAUN J L, et al. High-entropy fluorite oxides. Journal of the European Ceramic Society, 2018,38(10):3578-3584. |
| [42] | SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A, 1976,32(5):751-767. |
| [43] |
WRIGHT A J, WANG Q Y, HUANG C Y, et al. From high-entropy ceramics to compositionally-complex ceramics: a case study of fluorite oxides. Journal of the European Ceramic Society, 2020,40(5):2120-2129.
DOI URL |
| [44] | LIU Y C, JIA D C, ZHOU Y, et al. Zn0.1Ca0.1Sr0.4Ba0.4ZrO3: a non-equimolar multicomponent perovskite ceramic with low thermal conductivity. Journal of the European Ceramic Society, 2020,40(15):6272-6277. |
| [45] |
ARTINI C, PANI M, CARNASCIALI M M, et al. Structural features of Sm- and Gd-doped ceria studied by synchrotron X-ray diffraction and μ-Raman spectroscopy. Inorganic Chemistry, 2015,54(8):4126-4137.
DOI URL PMID |
| [46] | TOBY B H. EXPGUI, a graphical user interface for GSAS. Journal of Applied Crystallography, 2001,34(2):210-213. |
| [47] | CHEN K P, PEI X T, TANG L, et al. A five-component entropy-stabilized fluorite oxide. Journal of the European Ceramic Society, 2018,38(11):4161-4164. |
| [48] |
DRAGOE N, BéRARDAN D. Order emerging from disorder. Science, 2019,366(6465):573.
URL PMID |
| [49] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. Journal of Materials Science & Technology, 2019,35(8):1700-1705. |
| [50] | WRIGHT A J, WANG Q Y, KO S T, et al. Size disorder as a descriptor for predicting reduced thermal conductivity in medium- and high-entropy pyrochlore oxides. Scripta Materialia, 2020,181:76-81. |
| [51] | KURODA Y, HAMANO H, MORI T, et al. Specific adsorption behavior of water on a Y2O3 surface. Langmuir, 2000,16(17):6937-6947. |
| [52] | SPIRIDIGLIOZZI L, FERONE C, CIOFFI R, et al. Entropy-stabilized oxides owning fluorite structure obtained by hydrothermal treatment. Materials, 2000,16(17):6937-6947. |
| [53] | CHEN H, ZHAO Z F, XIANG H M, et al. Effect of reaction routes on the porosity and permeability of porous high entropy (Y0.2Yb0.2Sm0.2Nd0.2Eu0.2)B6 for transpiration cooling. Journal of Materials Science & Technology, 2020,38:80-85. |
| [54] | CUI S F, YANG W S, QIAN Z N. Research thermal decomposition fo lanthanum hydroxide by thermogravimetry. Chemical Journal of Chinese University, 1987,8(3):271-272. |
| [55] | SURYANARAYANA C. Mechanical alloying and milling. Progress in Materials Science, 2001,46(1/2):1-184. |
| [56] |
HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Materialia, 2019,166:271-280.
DOI URL |
| [1] | 李汪国, 刘佃光, 王珂玮, 马百胜, 刘金铃. 闪烧合成高熵氧化物陶瓷(MgCoNiCuZn)O的性能[J]. 无机材料学报, 2022, 37(12): 1289-1294. |
| [2] | 王义良, 艾云龙, 杨书伟, 梁炳亮, 郑振环, 欧阳晟, 何文, 陈卫华, 刘长虹, 张建军, 刘智勇. M3O4(M=FeCoCrMnMg)高熵氧化物粉体的简易制备及超电容性能研究[J]. 无机材料学报, 2021, 36(4): 425-430. |
| [3] | 巢亚军,原鲜霞,马紫峰,邓晓燕,于文利. 球磨工艺对炭气凝胶(CA)-SiO复合材料结构和电化学性能的影响[J]. 无机材料学报, 2008, 23(5): 917-922. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||