无机材料学报 ›› 2021, Vol. 36 ›› Issue (3): 292-298.DOI: 10.15541/jim20200340 CSTR: 32189.14.10.15541/jim20200340
杨言言1,2( ), 李永国3, 祝小雯1, 杜晓2, 马旭莉2, 郝晓刚2(
), 李永国3, 祝小雯1, 杜晓2, 马旭莉2, 郝晓刚2( )
)
收稿日期:2020-06-20
修回日期:2020-08-27
出版日期:2021-03-20
网络出版日期:2020-09-09
通讯作者:
郝晓刚, 教授. E-mail: xghao@tyut.edu.cn, tyutxghao@hotmail.com
作者简介:杨言言(1983-), 女, 博士, 讲师. E-mail: yangyy0927@163.com
基金资助:
YANG Yanyan1,2( ), LI Yongguo3, ZHU Xiaowen1, DU Xiao2, MA Xuli2, HAO Xiaogang2(
), LI Yongguo3, ZHU Xiaowen1, DU Xiao2, MA Xuli2, HAO Xiaogang2( )
)
Received:2020-06-20
Revised:2020-08-27
Published:2021-03-20
Online:2020-09-09
Contact:
HAO Xiaogang, professor. E-mail: xghao@tyut.edu.cn, tyutxghao@hotmail.com
About author:YANG Yanyan (1983-), female, PhD, lecturer. E-mail:yangyy0927@163.com
Supported by:摘要:
磷是植物体生长的重要营养素, 也是引发水体富营养化的重要因素, 因此废水中磷酸盐的去除与回收均至关重要。本研究采用单极脉冲电沉积法在炭布上制备镍钴双氢氧化物, 并于管式炉中原位焙烧制得镍钴双金属氧化物(NiCo-Layered Double Oxide, NiCo-LDO), 将其用于电控离子交换(Electrochemically Switched Ion Exchange, ESIX)过程实现PO4 3-的去除与回收。实验对比了ESIX与离子交换(Ion Exchange, IX)过程中NiCo-LDO对PO4 3-的去除性能, 并考察了其选择性及循环稳定性。结果表明, 在(10.00±0.05) mg/L的PO4 3-溶液中, ESIX过程中膜对PO4 3-的离子交换量约为IX的2倍; NiCo-LDO对PO4 3-具有高选择性, 且经过5次循环后, 离子交换量仍可达到初始值的92%以上; 结合XPS分析, 发现NiCo-LDO对PO4 3-的ESIX过程包括一个不可逆的“记忆效应”结构恢复过程及两个可逆的层板金属离子氧化/还原和PO4 3-与O-H基团的配体交换过程。
中图分类号:
杨言言, 李永国, 祝小雯, 杜晓, 马旭莉, 郝晓刚. 电活性镍钴双金属氧化物高选择性去除/回收水中磷酸盐离子[J]. 无机材料学报, 2021, 36(3): 292-298.
YANG Yanyan, LI Yongguo, ZHU Xiaowen, DU Xiao, MA Xuli, HAO Xiaogang. Potential Induced Reversible Removal/Recovery of Phosphate Anions with High Selectivity Using an Electroactive NiCo-layered Double Oxide Film[J]. Journal of Inorganic Materials, 2021, 36(3): 292-298.

图1 NiCo-LDH (a, c) 和NiCo-LDO (b, d)的SEM照片及NiCo-LDO的TEM照片(e)和HRTEM照片(f)
Fig. 1 SEM images of NiCo-LDH (a, c) and NiCo-LDO (b, d), TEM (e) and HRTEM (f) images of NiCo-LDO

图2 NiCo-LDH焙烧前后的XRD图谱(a)、NiCo-LDO的XPS全谱(b)、Co2p(c)及Ni2p(d)高分辨率XPS谱
Fig. 2 XRD patterns of NiCo-LDH and NiCo-LDO (a), full XPS (b), high resolution Co2p (c), and Ni2p (d) spectra of NiCo-LDO
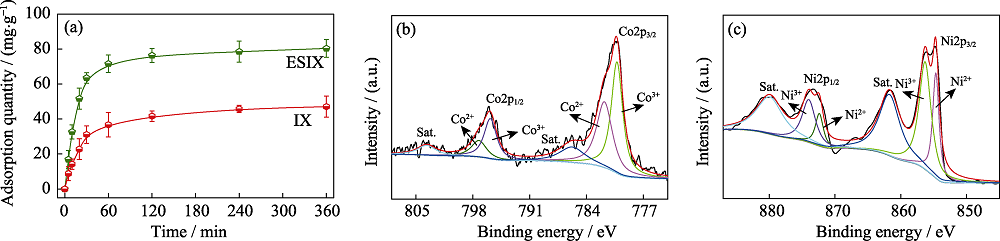
图3 NiCo-LDO在ESIX及IX过程中对PO43-的吸附曲线(a)、NiCo-LDO氧化后Co2p(b)及Ni2p(c)高分辨率XPS谱
Fig. 3 Kinetic adsorption curves of PO43- on NiCo-LDO under ESIX or IX process (a), high resolution Co2p (b) and Ni2p (c) spectra of NiCo-LDO after oxidation

图4 NiCo-LDO在四元混合溶液中对PO43-、SO42-、I-和Cl-的竞争吸附曲线
Fig. 4 Competitive adsorption kinetics curves of PO43-, SO42-, I-, and Cl- onto NiCo-LDO in 21.19 mg/L PO43-, 21.71 mg/L SO42-, 21.64 mg/L I-, and 22.85 mg/L Cl- mixed solution
| Anion-LDH | Ecp/(kJ•mol-1) |
|---|---|
| Cl--LDH | -455.88 |
| I--LDH | -345.20 |
| SO42--LDH | -813.90 |
| PO43--LDH | -868.36 |
表1 NiCo-LDH与阴离子之间结合能计算结果
Table 1 Calculated binding energy between anion-and NiCo-LDH
| Anion-LDH | Ecp/(kJ•mol-1) |
|---|---|
| Cl--LDH | -455.88 |
| I--LDH | -345.20 |
| SO42--LDH | -813.90 |
| PO43--LDH | -868.36 |
| Ion adsorbent | Adsorption quantity/(mg•g-1) | Time/h | Ref. |
|---|---|---|---|
| Aluminum oxide hydroxide | 36.27 | 4 | [6] |
| Fe-Mn binary oxide | 33.2 | 24 | [29] |
| Hydroxy-aluminum | 12.7 | 7 | [30] |
| MgFe-Zr-LDH@magnetic particles | 30 | 24 | [31] |
| Am-ZrO2 | 67.29 | 8 | [32] |
| Mg-Fe-Cl LDH | 9.8 | 6 | [10] |
| Nano-La(III) (hydr)oxides | ~55 | 10 | [33] |
| La(OH)3/Fe3O4 | 83.5 | 5 | [5] |
| NiCo-LDO | 159.36 | 6 | This work |
表2 文献报道的PO43-吸附剂对PO43-吸附量的对比
Table 2 Comparison of the ion adsorption quantity with those reported PO43- ion adsorbents
| Ion adsorbent | Adsorption quantity/(mg•g-1) | Time/h | Ref. |
|---|---|---|---|
| Aluminum oxide hydroxide | 36.27 | 4 | [6] |
| Fe-Mn binary oxide | 33.2 | 24 | [29] |
| Hydroxy-aluminum | 12.7 | 7 | [30] |
| MgFe-Zr-LDH@magnetic particles | 30 | 24 | [31] |
| Am-ZrO2 | 67.29 | 8 | [32] |
| Mg-Fe-Cl LDH | 9.8 | 6 | [10] |
| Nano-La(III) (hydr)oxides | ~55 | 10 | [33] |
| La(OH)3/Fe3O4 | 83.5 | 5 | [5] |
| NiCo-LDO | 159.36 | 6 | This work |
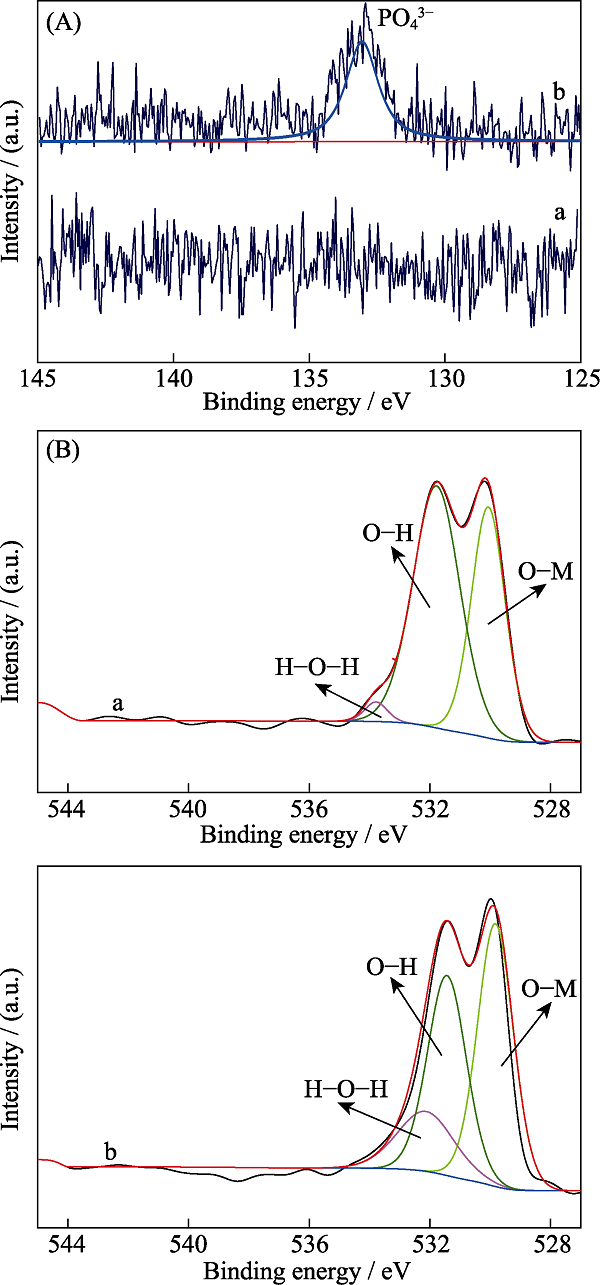
图6 NiCo-LDO氧化前(曲线组a)后(曲线组b)P2p(A)及O1s(B)的高分辨率XPS图谱
Fig. 6 High resolution P2p (A) and O1s (B) spectra of NiCo-LDO before (curves a) and after (curves b) oxidation
| [1] | WANG XIANG-XUE, YU SHU-JUN, WANG XIANG-KE . Removal of radionuclides by metal-organic framework-based materials. Journal of Inorganic Materials, 2019,34(1):17-26. |
| [2] | WANG XIANG-XUE, LI XING, WANG JIA-QI , et al. Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution. Journal of Inorganic Materials, 2020,35(3):260-270. |
| [3] |
XIONG W, TONG J, YANG Z , et al. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: performance and mechanism. Journal of Colloid and Interface Science, 2017,493:17-23.
DOI URL PMID |
| [4] | ZHANG XIAO-FENG, ZHANG GUAN-HUA, MENG YUE , et al. Photocatalytic degradation of methylene blue by Schiff-base cobalt modified CoCr layered double hydroxides. Journal of Inorganic Materials, 2019,34(9):974-982. |
| [5] |
WU B, FANG L, FORTNER J D , et al. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Research, 2017,126:179-188.
DOI URL PMID |
| [6] |
TANADA S, KABAYAMA M, KAWASAKI N , et al. Removal of phosphate by aluminum oxide hydroxide. Journal of Colloid and Interface Science, 2003,257(1):135-140.
DOI URL PMID |
| [7] |
WU B, WAN J, ZHANG Y , et al. Selective phosphate removal from water and wastewater using worption: process fundamentals and removal mechanisms. Environmental Science & Technology, 2020,54(1):50-66.
DOI URL PMID |
| [8] | MAYER B, GERRITY D, RITTMANN B , et al. Innovative strategies to achieve low total phosphorus concentrations in high water flows. Critical Reviews in Environmental Science & Technology, 2013,43(4):409-441. |
| [9] | LIU R, CHI L, WANG X , et al. Review of metal(hydr)oxide and other adsorptive materials for phosphate removal from water. Journal of Environmental Chemical Engineering, 2018,6(4):5269-5286. |
| [10] | ASHEKUZZAMAN S, JIANG J . Strategic phosphate removal/ recovery by a reusable Mg-Fe-Cl layered double hydroxide. Process Safety and Environmental Protection, 2017,107:454-462. |
| [11] | XIAO JUN-QIANG, HAO XIAO-GANG . Electrochemically switched ion exchange. Progress in Chemistry, 2010,22(12):2420-2427. |
| [12] | LIAO S, XUE C, WANG Y , et al. Simultaneous separation of iodide and cesium ions from dilute wastewater based on PPy/PTCF and NiHCF/PTCF electrodes using electrochemically switched ion exchange method. Separation and Purification Technology, 2015,139:63-69. |
| [13] | JU JIAN, HAO XIAO-GANG, ZHANG ZHONG-LIN , et al. Electrochemically controlled ion separation performances of electrodeposited nickel hexacyanoferrate thin films in alkaline earth metal solution. Journal of Inorganic Materials, 2008,23(6):1115-1120. |
| [14] |
DU X, ZHANG H, HAO X , et al. Facile preparation of ion- imprinted composite film for selective electrochemical removal of nickel (II) ions. ACS Applied Materials & Interfaces, 2014,6(12):9543-9549.
DOI URL PMID |
| [15] |
ZHANG Q, DU X, MA X , et al. Facile preparation of electroactive amorphous α-ZrP/PANI hybrid film for potential-triggered adsorption of Pb2+ ions. Journal of Hazardous Materials, 2015,289:91-100.
DOI URL PMID |
| [16] | DU X, SUN X, ZHANG H , et al. A facile potential-induced in-situ ion removal trick: fabrication of high-selective ion imprinted film for trivalent yttrium ion separation. Electrochimica Acta, 2015,176:1313-1323. |
| [17] | YANG Y, DU X, AN X , et al. Potential-induced reversible uptake/release of perchlorate from wastewater by polypyrrole@CoNi-layered double hydroxide modified electrode with proton- ligand effect. Journal of Colloid and Interface Science, 2018,523:159-168. |
| [18] | YANG Y, DU X, ABUDULA A , et al. Highly efficient defluoridation using a porous MWCNT@NiMn-LDH composites based on ion transport of EDL coupled with ligand exchange mechanism. Separation and Purification Technology, 2019,223:154-161. |
| [19] | LI M, LIU J, XU Y , et al. Phosphate adsorption on metal oxides and metal hydroxides: a comparative review. Environmental Reviews, 2016,24(3):319-332. |
| [20] | PANG HONG-WEI, TANG HAO, WANG JIA-QI , et al. Ternary layered double hydroxide supported sulfide NZVI: efficient U(VI) elimination and mechanism. Journal of Inorganic Materials, 2020,35(3):381-389. |
| [21] |
SHAO M, LI Z, ZHANG R , et al. Hierarchical conducting polymer@clay core-shell arrays for flexible all-solid-state supercapacitor devices. Small, 2015,11(29):3530-3538.
DOI URL PMID |
| [22] | YU C, ZHANG L, SHI J , et al. A simple template-free strategy to synthesize nanoporous manganese and nickel oxides with narrow pore size distribution, and their electrochemical properties. Advanced Functional Materials, 2008,18(10):1544-1554. |
| [23] | TASKOPRU T, BAYANSAL F, ŞAHIN B , et al. Structural and optical properties of Co-doped NiO films prepared by SILAR method. Philosophical Magazine, 2015,95(1):32-40. |
| [24] | CHEN H, HU L, CHEN M , et al. Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Advanced Functional Materials, 2014,24(7):934-942. |
| [25] |
MANIVASAKAN P, RAMASAMY P, KIM J . Use of urchin-like Ni(x)Co(3-x)O4 hierarchical nanostructures based on non-precious metals as bifunctional electrocatalysts for anion-exchange membrane alkaline alcohol fuel cells. Nanoscale, 2014,6(16):9665-9672.
URL PMID |
| [26] |
SUN B, HAO X, Wang Z , et al. Separation of low concentration of cesium ion from wastewater by electrochemically switched ion exchange method: experimental adsorption kinetics analysis. Journal of Hazardous Materials, 2012,233:177-183.
DOI URL PMID |
| [27] |
CAI J, ZHANG Y, PAN B , et al. Efficient defluoridation of water using reusable nanocrystalline layered double hydroxides impregnated polystyrene anion exchanger. Water Research, 2016,102:109-116.
DOI URL PMID |
| [28] | GOH K, LIM T, BANAS A , et al. Sorption characteristics and mechanisms of oxyanions and oxyhalides having different molecular properties on Mg/Al layered double hydroxide nanoparticles. Journal of Hazardous Materials, 2010,179(1/2/3):818-827. |
| [29] |
ZHANG G, LIU H, LIU R , et al. Removal of phosphate from water by a Fe-Mn binary oxide adsorbent. Journal of Colloid and Interface Science, 2009,335(2):168-174.
DOI URL PMID |
| [30] | YAN L, XU Y, YU H , et al. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron- aluminum pillared bentonites. Journal of Hazardous Materials, 2010,179(1/2/3):244-250. |
| [31] |
DRENKOVA-TUHTAN A, MANDEL K, PAULUS A , et al. Phosphate recovery from wastewater using engineered superparamagnetic particles modified with layered double hydroxide ion exchangers. Water Research, 2013,47(15):5670-5677.
DOI URL PMID |
| [32] |
SU Y, CUI H, LI Q , et al. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Research, 2013,47(14):5018-5026.
DOI URL PMID |
| [33] | QIU H, LIANG C, YU J , et al. Preferable phosphate sequestration by nano-La(III)(hydr)oxides modified wheat straw with excellent properties in regeneration. Chemical Engineering Journal, 2017,315:345-354. |
| [1] | 万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503. |
| [2] | 吕昕怿, 相恒阳, 曾海波. 长程有序助力钙钛矿QLED高性能化[J]. 无机材料学报, 2025, 40(1): 111-112. |
| [3] | 陈梦杰, 王倩倩, 吴成铁, 黄健. 基于DFT的描述符预测生物陶瓷的降解性[J]. 无机材料学报, 2024, 39(10): 1175-1181. |
| [4] | 李乾利, 黎乃鑫, 李育成, 刘慎业, 程帅, 杨光, 任宽, 王峰, 赵景泰. 辐射光致发光材料及其应用研究进展[J]. 无机材料学报, 2023, 38(7): 731-749. |
| [5] | 马鹏飞, 李日红, 张 龙. 溶胶-凝胶法制备高比表面积铝磷钙生物活性玻璃[J]. 无机材料学报, 2017, 32(1): 107-112. |
| [6] | 徐永春, 陈丹平, 李顺光, 胡丽丽, 唐景平. 磷酸盐激光玻璃中杂质离子铁和铜对激光效率的影响[J]. 无机材料学报, 2015, 30(3): 240-244. |
| [7] | 刘 斌, 董寅生, 吴红艳, 苏 静, 林萍华, 郭宗科. 多次涂覆复合磷酸盐多孔陶瓷的表面结构和细胞相容性[J]. 无机材料学报, 2014, 29(2): 179-184. |
| [8] | 张 磊, 黄 利, 丁 佳, 陈辉宇, 陈 伟, 胡丽丽. 酸碱处理对磷酸盐激光玻璃表面的侵蚀研究[J]. 无机材料学报, 2012, 27(6): 627-632. |
| [9] | 刘 斌, 董寅生, 林萍华, 张 俊, 苏 静. 复合磷酸盐多孔生物陶瓷的制备及体外细胞相容性[J]. 无机材料学报, 2011, 26(7): 759-764. |
| [10] | 杨 刚,钱 奇,杨中民. P2O5-BaO-Al2O3-K2O磷酸盐激光玻璃的表面处理[J]. 无机材料学报, 2010, 15(2): 196-200. |
| [11] | 王中俭,吴纬,姜波,胡一晨,王少波. ZnO-MgO-P2O5磷酸盐玻璃酸溶机理研究[J]. 无机材料学报, 2008, 23(1): 155-158. |
| [12] | 孙建之,邓小川,宋士涛,李法强,马培华. 新型特效Na离子吸附剂Li1+xAlxTi2-x(PO4)3的制备和性能研究[J]. 无机材料学报, 2006, 21(1): 169-175. |
| [13] | 杨钢锋,赵三银,邓再德,孙家森,姜中宏. 掺铒磷酸盐玻璃反应气氛法除水的研究[J]. 无机材料学报, 2005, 20(5): 1083-1088. |
| [14] | 段成军,吴雪艳,陈昊鸿,杨昕昕,赵景泰. Ba3BPO7的合成及其水解、热稳定性和稀土掺杂发光性能的研究[J]. 无机材料学报, 2005, 20(5): 1043-1048. |
| [15] | 赵宏生,李艳青,周万城,罗发,唐春和. MoO3-V2O5-P2O5-Fe2O3玻璃的制备及性能研究[J]. 无机材料学报, 2005, 20(3): 563-569. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||