无机材料学报 ›› 2020, Vol. 35 ›› Issue (6): 709-716.DOI: 10.15541/jim20190426 CSTR: 32189.14.10.15541/jim20190426
所属专题: 结构陶瓷论文精选(2020)
收稿日期:2019-08-14
修回日期:2019-10-29
出版日期:2020-06-20
网络出版日期:2019-12-29
作者简介:王志虎(1978-), 男, 博士研究生, 高级工程师. E-mail: zhihu_wang@163.com;基金资助:
WANG Zhihu1,ZHANG Jumei2,BAI Lijing1,ZHANG Guojun1( )
)
Received:2019-08-14
Revised:2019-10-29
Published:2020-06-20
Online:2019-12-29
Supported by:摘要:
采用不同浓度的NaOH溶液对AZ31镁合金微弧氧化(Micro-arc oxidation, MAO)陶瓷层进行水热处理, 研究了水热溶液浓度对MAO陶瓷层组织结构及耐蚀性能的影响, 探讨了水热成膜及膜层的腐蚀机理。研究结果表明:水热处理过程中MAO陶瓷层表面的MgO部分溶解, 释放出的Mg 2+与水热溶液中的OH -结合形成Mg(OH)2纳米片沉淀在陶瓷层表面及孔洞内。随着水热溶液中NaOH浓度的增加, 水热处理过程中形成的Mg(OH)2将MAO陶瓷层表面的孔洞及裂纹等固有缺陷闭合, 提高了膜层的致密性。电化学实验结果表明, MAO及水热复合处理所制备的Mg(OH)2/MAO复合膜层比单一MAO陶瓷层具有更好的耐蚀性, 而且随着NaOH浓度的提高, Mg(OH)2/MAO复合膜层的耐蚀性增强; 浸泡实验结果表明Mg(OH)2/MAO复合膜层能为镁合金基体提供长久的腐蚀防护保护能力。
中图分类号:
王志虎,张菊梅,白力静,张国君. AZ31镁合金微弧氧化陶瓷层表面Mg(OH)2膜层的制备及耐蚀性[J]. 无机材料学报, 2020, 35(6): 709-716.
WANG Zhihu,ZHANG Jumei,BAI Lijing,ZHANG Guojun. Mg(OH)2 Film on Micro-arc Oxidation Ceramic Coating of AZ31 Magnesium Alloy: Preparation and Corrosion Resistance[J]. Journal of Inorganic Materials, 2020, 35(6): 709-716.
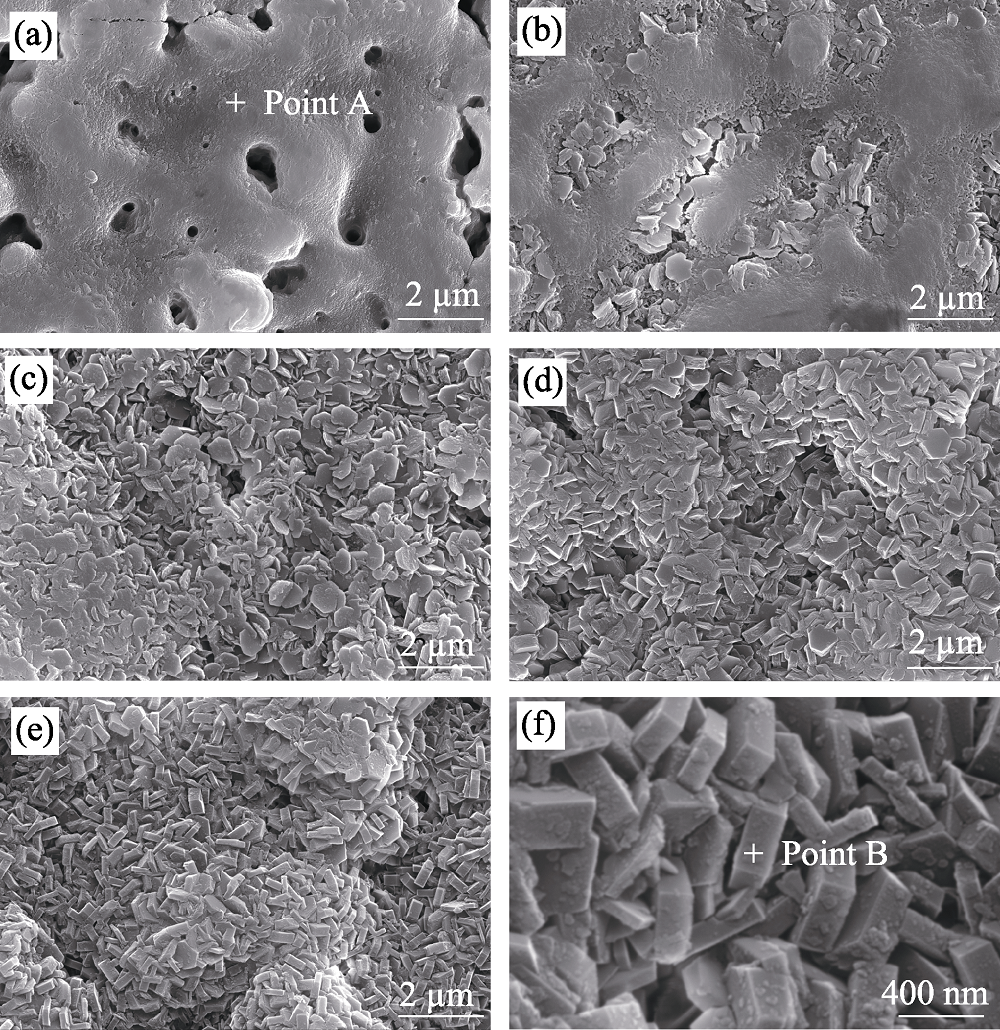
图2 MAO陶瓷层和HT/MAO复合膜层的表面形貌
Fig. 2 Surface morphologies of MAO coating and HT/MAO composite coating (a) MAO; (b) HT-0/MAO; (c) HT-0.2/MAO; (d) HT-0.5/MAO; (e) HT-2.0/MAO; (f) HT-2.0/MAO in high magnification
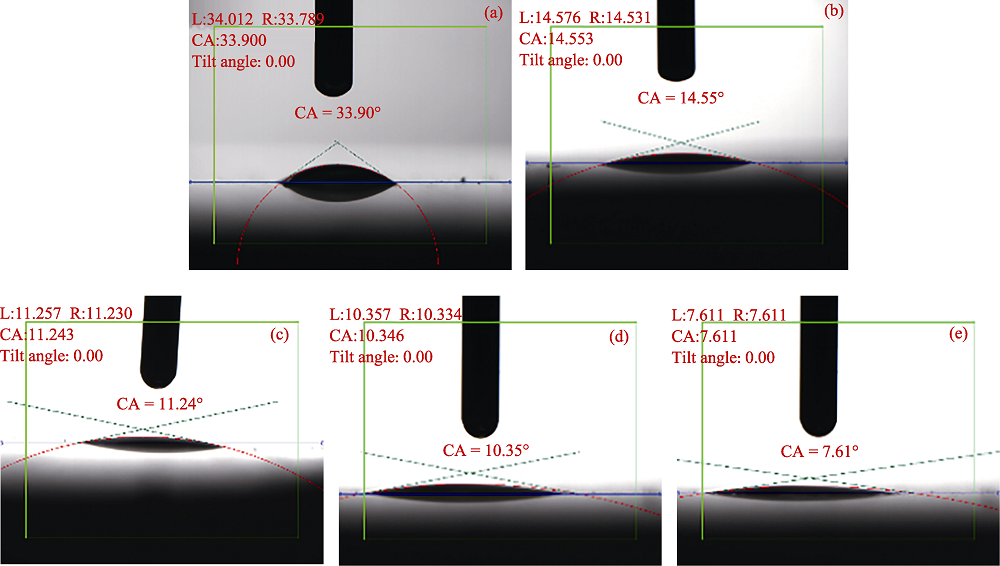
图4 MAO陶瓷层和HT/MAO复合膜层的静态接触角
Fig. 4 Static contact angles of MAO coating and HT/MAO composite coating (a)MAO; (b) HT-0/MAO; (c)HT-0.2/MAO; (d)HT-0.5/MAO; (e)HT-2.0/MAO
| Samples | Ecorr /V | Icorr /(μA·cm-2) | βa /V | βc/V | RP/(kΩ·cm2) | CR/(mm·a-1) |
|---|---|---|---|---|---|---|
| Bare AZ31 | -1.460 | 22.34 | 0.284 | 0.395 | 3.211 | 510.5×10-3 |
| MAO | -1.383 | 0.0530 | 1.340 | 0.209 | 1.481×103 | 1.211×10-3 |
| HT-0.2/MAO | -1.376 | 0.0345 | 0.938 | 0.409 | 3.585×103 | 0.788×10-3 |
| HT-2.0/MAO | -1.350 | 0.0187 | 1.226 | 0.208 | 4.129×103 | 0.427×10-3 |
表1 动电位极化曲线拟合参数
Table 1 Fitting parameters derived from the potentiodynamic polarization curves
| Samples | Ecorr /V | Icorr /(μA·cm-2) | βa /V | βc/V | RP/(kΩ·cm2) | CR/(mm·a-1) |
|---|---|---|---|---|---|---|
| Bare AZ31 | -1.460 | 22.34 | 0.284 | 0.395 | 3.211 | 510.5×10-3 |
| MAO | -1.383 | 0.0530 | 1.340 | 0.209 | 1.481×103 | 1.211×10-3 |
| HT-0.2/MAO | -1.376 | 0.0345 | 0.938 | 0.409 | 3.585×103 | 0.788×10-3 |
| HT-2.0/MAO | -1.350 | 0.0187 | 1.226 | 0.208 | 4.129×103 | 0.427×10-3 |
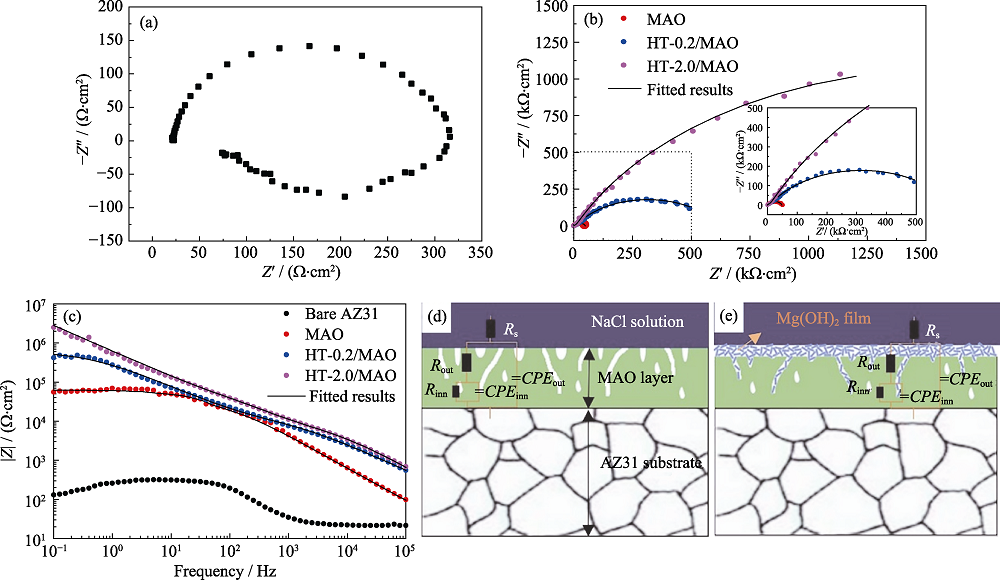
图6 AZ31和带有不同膜层试样的Nyquist图、Bode图以及等效电路图
Fig. 6 Nyquist and Bode plots of bare AZ31 and coated samples as well as equivalent circuit (a) Nyquist plots of bare AZ31; (b) Nyquist plots of coated samples; (c) Bode plots of all samples; (d-e) physical model and equivalent circuit for coated samples
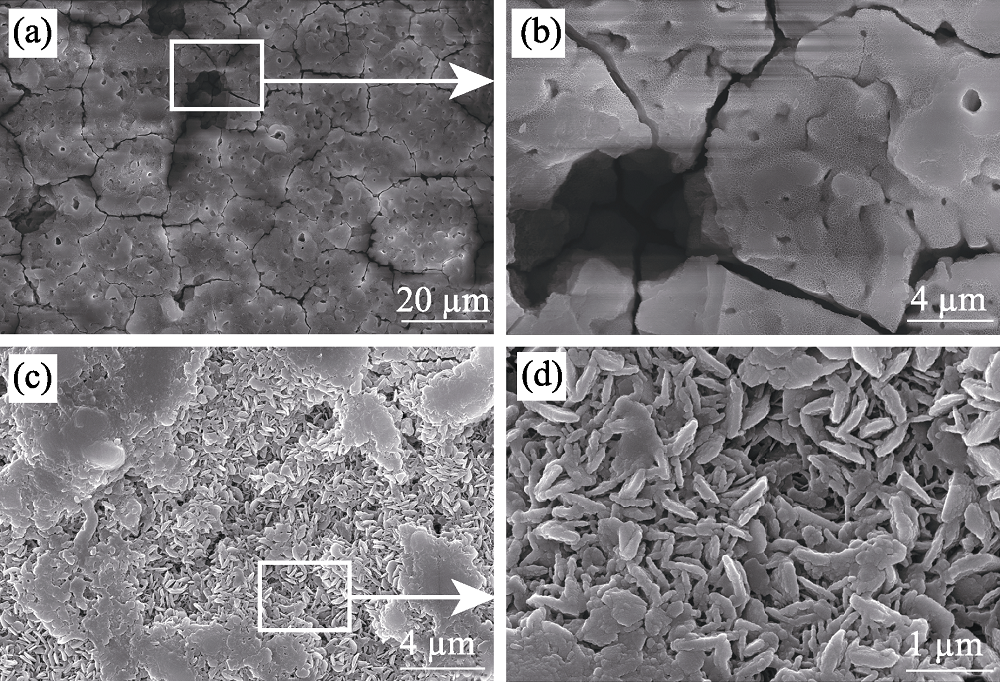
图9 MAO和HT-0.2/MAO试样在3.5wt% NaCl溶液中浸泡14 d后的表面形貌
Fig. 9 Surface morphologies of MAO and HT-0.2 /MAO after immersion in 3.5wt% NaCl solution for 14 d (a-b) MAO; (c-d) HT-0.2/MAO
| [1] |
SANKARE NARAYANAN T S N, PARK Il S, LEE M H . Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: prospects and challenges. Prog. Mater. Sci., 2014,60:1-71.
DOI URL |
| [2] |
ZHAO J M, XIE X, ZHANG C . Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci., 2017,114:146-155.
DOI URL |
| [3] |
ZHU Y Y, ZHANG S F, ZHAO R F , et al. Influences of Na2SiO3 and EDTA-ZnNa2 concentration on properties of zinc-containing coatings on WE43 magnesium alloys. Surf. Coat. Technol., 2018,356:108-122.
DOI URL |
| [4] |
LU X P, BLAWERT C, HUANG Y D , et al. Plasma electrolytic oxidation coatings on Mg alloy with addition of SiO2 particles. Electrochim. Acta, 2016,187:20-33.
DOI URL |
| [5] |
TOORANI M, ALIOFKHAZRAEI M, SABOUR ROUHAGHDAM A . Microstructural, protective, inhibitory and semiconducting properties of PEO coatings containing CeO2 nanoparticles formed on AZ31 Mg alloy. Surf. Coat. Technol., 2018,352:561-580.
DOI URL |
| [6] |
BORDBAR-KHIABANI A, YARMAND B, MOZAFARI M . Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol., 2019,360:153-171.
DOI URL |
| [7] |
BAI L J, DONG B X, CHEN G T , et al. Effect of positive pulse voltage on color value and corrosion property of magnesium alloy black micro-arc oxidation ceramic coating. Surf. Coat. Technol., 2019,374:402-408.
DOI URL |
| [8] |
EZHILSELVI V, NITHIN J, BALARAJU J N , et al. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol., 2016,288:221-229.
DOI URL |
| [9] |
LYU G H, CHEN H, GU W C , et al. Effects of current frequency on the structural characteristics and corrosion property of ceramic coatings formed on magnesium alloy by PEO technology. J. Mater. Process. Tech., 2008,208(1/2/3):9-13.
DOI URL |
| [10] |
ZOU B, LÜ G H, ZHANG G L , et al. Effect of current frequency on properties of coating formed by microarc oxidation on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China, 2015,25(5):1500-1505.
DOI URL |
| [11] |
CHANG L R, CAO F H, CAI J S , et al. Influence of electric parameters on MAO of AZ91D magnesium alloy using alternative square-wave power source. Trans. Nonferrous Met. Soc. China, 2011,21(2):307-316.
DOI URL |
| [12] |
GU Y H, BANDOPADHYAY S, CHEN C F , et al. Effect of oxidation time on the corrosion behavior of micro-arc oxidation produced AZ31 magnesium alloys in simulated body fluid. J. Alloys Compd., 2012,543:109-117.
DOI URL |
| [13] |
GNEDENKOV S V, SINEBRYUKHOV S L, MASHTALYAR D V , et al. Composite coatings formed on the PEO-layers with the use of solutions of tetrafluoroethylene telomers. Surf. Coat. Technol., 2018,346:53-62.
DOI URL |
| [14] |
LUO D, LIU Y, YIN X M , et al. Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mg-5Sn-1Zn alloy substrate. J. Alloys Compd., 2018,731:731-738.
DOI URL |
| [15] |
CUI L Y, LIU H P, ZHANG W L , et al. Corrosion resistance of a superhydrophobic micro-arc oxidation coating on Mg-4Li-1Ca alloy. J. Mater. Sci. Technol., 2017,33(11):1263-1271.
DOI URL |
| [16] |
QIU Z Z, SUN J, WANG R , et al. Magnet-induced fabrication of a superhydrophobic surface on ZK60 magnesium alloy. Surf. Coat. Technol., 2016,286:246-250.
DOI URL |
| [17] |
ZHU Y Y, WU G M, ZHANG Y H , et al. Growth and characterization of Mg(OH)2 film on magnesium alloy AZ31. Appl. Surf. Sci., 2011, 257(14):6129-6137.
DOI URL |
| [18] |
WANG C M, SHEN J, ZHANG X K , et al. In vitrodegradation and cytocompatibility of a silane/Mg(OH)2 composite coating on AZ31 alloy by spin coating. J. Alloys Compd., 2017,714:186-193.
DOI URL |
| [19] |
JIN Q, TIAN G Y, LI J X , et al. The study on corrosion resistance of superhydrophobic magnesium hydroxide coating on AZ31B magnesium alloy. Colloid. Surface A, 2019,577:8-16.
DOI URL |
| [20] | JEONG H, YOO Y . Synthesis and characterization of thin films on magnesium alloy using a hydrothermal method. Surf. Coat. Technol., 2015,284:26-30. |
| [21] |
ZHU Y Y, ZHAO Q, ZHANG Y H , et al. Hydrothermal synthesis of protective coating on magnesium alloy using de-ionized water. Surf. Coat. Technol., 2012,206(11/12):2961-2966.
DOI URL |
| [22] | LI C Y, FAN X L, ZENG R C , et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31—influence of EDTA. J. Mater. Sci. Technol., 2019,35(6):1088-1098. |
| [23] |
AMARAL L F, OLIVEIRA I R, BONADIA P , et al. Chelants to inhibit magnesia (MgO) hydration. Ceram. Int., 2011,37(5):1537-1542.
DOI URL |
| [24] |
DUAN H P, DU K Q, YAN C W , et al. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim. Acta, 2006,51(14):2898-2908.
DOI URL |
| [25] |
CUI L Y, ZENG R C, GUAN S K , et al. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: the influence of porosity. J. Alloys Compd., 2017,695:2464-2476.
DOI URL |
| [26] |
FENG J, CHEN Y, LIU X H , et al. In-situ hydrothermal crystallization Mg(OH)2 films on magnesium alloy AZ91 and their corrosion resistance properties. Mater. Chem. Phys., 2013,143(1):322-329.
DOI URL |
| [27] |
ZHANG G, WU L, TANG A T , et al. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci., 2018,139:370-382.
DOI URL |
| [28] |
LIU Y, YIN X M, ZHANG J J , et al. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta, 2014,125:395-403.
DOI URL |
| [29] |
ZHANG G, WU L, TANG A T , et al. A novel approach to fabricate protective layered double hydroxide films on the surface of anodized Mg-Al alloy. Adv. Mater. Interfaces, 2017,4(12):1700163.
DOI URL |
| [1] | 杜佳恒, 范鑫丽, 肖东琴, 尹一然, 李忠, 贺葵, 段可. 电泳沉积制备微弧氧化钛表面氧化镁涂层及其生物学性能[J]. 无机材料学报, 2023, 38(12): 1441-1448. |
| [2] | 杨少辉, 闫淑芳, 李世江, 陈伟东, 杜培, 马文. 阶段占空比对ZrH1.8表面微弧氧化陶瓷层性能的影响[J]. 无机材料学报, 2020, 35(10): 1112-1116. |
| [3] | 杜培, 闫淑芳, 陈伟东, 李世江, 马文. 石墨烯浓度对ZrH1.8表面微弧氧化陶瓷层的影响[J]. 无机材料学报, 2019, 34(11): 1175-1180. |
| [4] | 刘小元, 刘宝丹, 姜亚南, 王柯, 周洋, 杨兵, 张兴来, 姜辛. 形貌可控及光学吸收性能可调的钙钛矿型SrTiO3纳米结构的原位生长[J]. 无机材料学报, 2019, 34(1): 65-71. |
| [5] | 张鹏飞, 闫淑芳, 陈伟东, 李世江, 耿艳花, 王宏兴. 正向电压对氢化锆微弧氧化阻氢膜层性能的影响[J]. 无机材料学报, 2018, 33(7): 793-797. |
| [6] | 张鹏飞, 闫淑芳, 陈伟东, 李世江, 赵丽, 王宏兴. 铝酸盐体系下反应时间对ZrH1.8表面阻氢膜层制备的影响[J]. 无机材料学报, 2018, 33(3): 284-288. |
| [7] | 王学政, 王海滨, 刘雪梅, 杨 涛, 宋晓艳. 抑制剂对WC-Co硬质合金涂层性能的影响[J]. 无机材料学报, 2017, 32(8): 813-818. |
| [8] | 罗军明, 吴小红, 徐吉林. 电解液组分对TiCP/Ti6Al4V复合材料微弧氧化膜耐蚀性及耐磨性影响[J]. 无机材料学报, 2017, 32(4): 418-424. |
| [9] | 李航, 卢松涛, 秦伟, 吴晓宏. 电流密度对MgO-ZnO陶瓷薄膜结构和热控性能的影响[J]. 无机材料学报, 2017, 32(12): 1292-1298. |
| [10] | 叶作彦, 刘道新, 李重阳, 张晓化, 张小明, 雷明霞. 封闭对铝合金微弧氧化膜在酸性溶液中耐蚀性的影响[J]. 无机材料学报, 2015, 30(6): 627-632. |
| [11] | 喻 杰, 韦东波, 王 岩, 吕鹏翔, 狄士春. 激光重熔改性铝合金微弧氧化膜层的组织与性能[J]. 无机材料学报, 2013, 28(8): 859-863. |
| [12] | 吕鹏翔, 韦东波, 郭成波, 李兆龙, 狄士春. 2024铝合金表面扫描式微弧氧化工艺研究[J]. 无机材料学报, 2013, 28(4): 381-386. |
| [13] | 薛文斌, 鲁 亮, 杜建成, 华 铭, 赵衍华. 2219铝合金搅拌摩擦焊接头微观组织对微弧氧化膜生长的影响[J]. 无机材料学报, 2011, 26(9): 897-901. |
| [14] | 王晓波, 田修波, 巩春志, 杨士勤. 镁合金微弧氧化Na2CO3诱导析气反应及结构调制[J]. 无机材料学报, 2011, 26(7): 721-725. |
| [15] | 潘应君, 宣圣柱, 谭 密, 周青春. 电容器用TiCN/Al复合铝箔的制备及其耐蚀性研究[J]. 无机材料学报, 2010, 25(9): 975-978. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||