无机材料学报 ›› 2020, Vol. 35 ›› Issue (1): 131-138.DOI: 10.15541/jim20190139 CSTR: 32189.14.10.15541/jim20190139
所属专题: MAX相和MXene材料; 副主编黄庆研究员专辑; MXene材料专辑(2020~2021); 【虚拟专辑】层状MAX,MXene及其他二维材料
马保凯1,2,3,李勉3,张绫芷2( ),翁新楚1,沈彩3,黄庆3
),翁新楚1,沈彩3,黄庆3
收稿日期:2019-03-28
出版日期:2020-01-20
网络出版日期:2019-05-29
作者简介:马保凯(1992-), 男, 硕士研究生. E-mail: mabaokai@nimte.ac.cn
MA Bao-Kai1,2,3,LI Mian3,CHEONG Ling-Zhi2( ),WENG Xin-Chu1,SHEN Cai3,HUANG Qing3
),WENG Xin-Chu1,SHEN Cai3,HUANG Qing3
Received:2019-03-28
Published:2020-01-20
Online:2019-05-29
About author:MA Bao-Kai (1992-), male, Master candidate. E-mail: mabaokai@nimte.ac.cn
Supported by:摘要:
本研究合成了具有垂直栅栏结构的二维MXene材料, 与辣根过氧化物酶进行固定, 构筑了过氧化氢电化学酶传感器。合成的MXene纳米栅栏具有大的比表面积, 优良的电子传导特性和在水溶液中的良好分散特性; 固定化在酶电极上的辣根过氧化物酶分子表现出了优良的过氧化氢催化效果。结果表明HRP@MXene/chitosan/GCE酶电化学传感器在过氧化氢浓度为5~1650 μmol/L范围内表现出很好的线性关系, 最低检测限为0.74 μmol/L, 且具有很好的操作稳定性, 该生物传感器被成功地应用于固态与液态食品中过氧化氢残留检测。
中图分类号:
马保凯, 李勉, 张绫芷, 翁新楚, 沈彩, 黄庆. 酶-二维MXene复合材料的制备及其电化学检测H2O2的应用[J]. 无机材料学报, 2020, 35(1): 131-138.
MA Bao-Kai, LI Mian, CHEONG Ling-Zhi, WENG Xin-Chu, SHEN Cai, HUANG Qing. Enzyme-MXene Nanosheets: Fabrication and Application in Electrochemical Detection of H2O2[J]. Journal of Inorganic Materials, 2020, 35(1): 131-138.
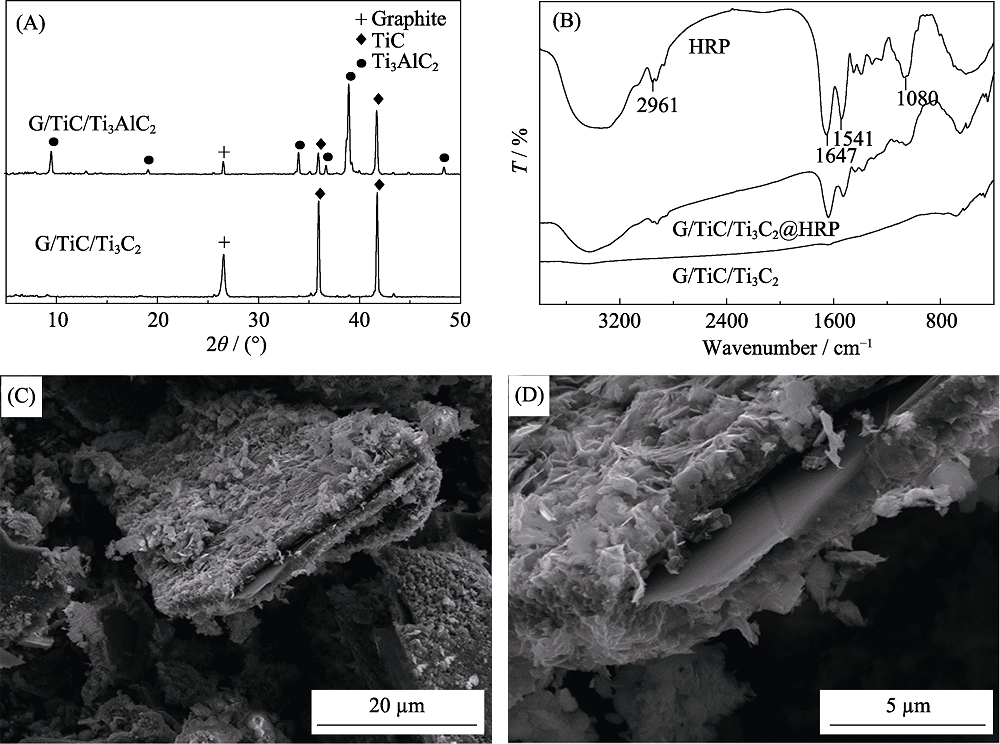
Fig. 2 XRD patterns of G/TiC/Ti3AlC2 and G/TiC/Ti3C2 (A); FT-IR spectra of the MXene, HRP and HRP@MXene (B); SEM images of the MXene G/TiC (C) and Ti3C2 (D)

Fig. S1 EIS of various electrodes in 0.1 mol?L-1 KCL aqueous solution containing 5 mmol?L-1 [Fe(CN)6]3-/4-: Chit (pH 5.0)/GCE (curve b, red line), Chit (pH 6.0)/GCE (curve c, blue line) , Chit (pH 6.5)/GCE (curve d, green line), Chit (pH 7.0)/GCE (curve e, pink line) (A); CV curves of Chit (pH 5.0)/GCE (curve b, red line), Chit (pH 6.0)/GCE (curve c, blue line) , Chit (pH 6.5)/GCE (curve d, green line) , Chit (pH 7.0)/GCE (curve e, pink line) electrodes cycled in 0.1 mol?L-1 KCL aqueous solution containing 5 mmol?L-1 [Fe(CN)6]3-/4-: (potential window: -0.1-0.5 V vs. SCE) (B)
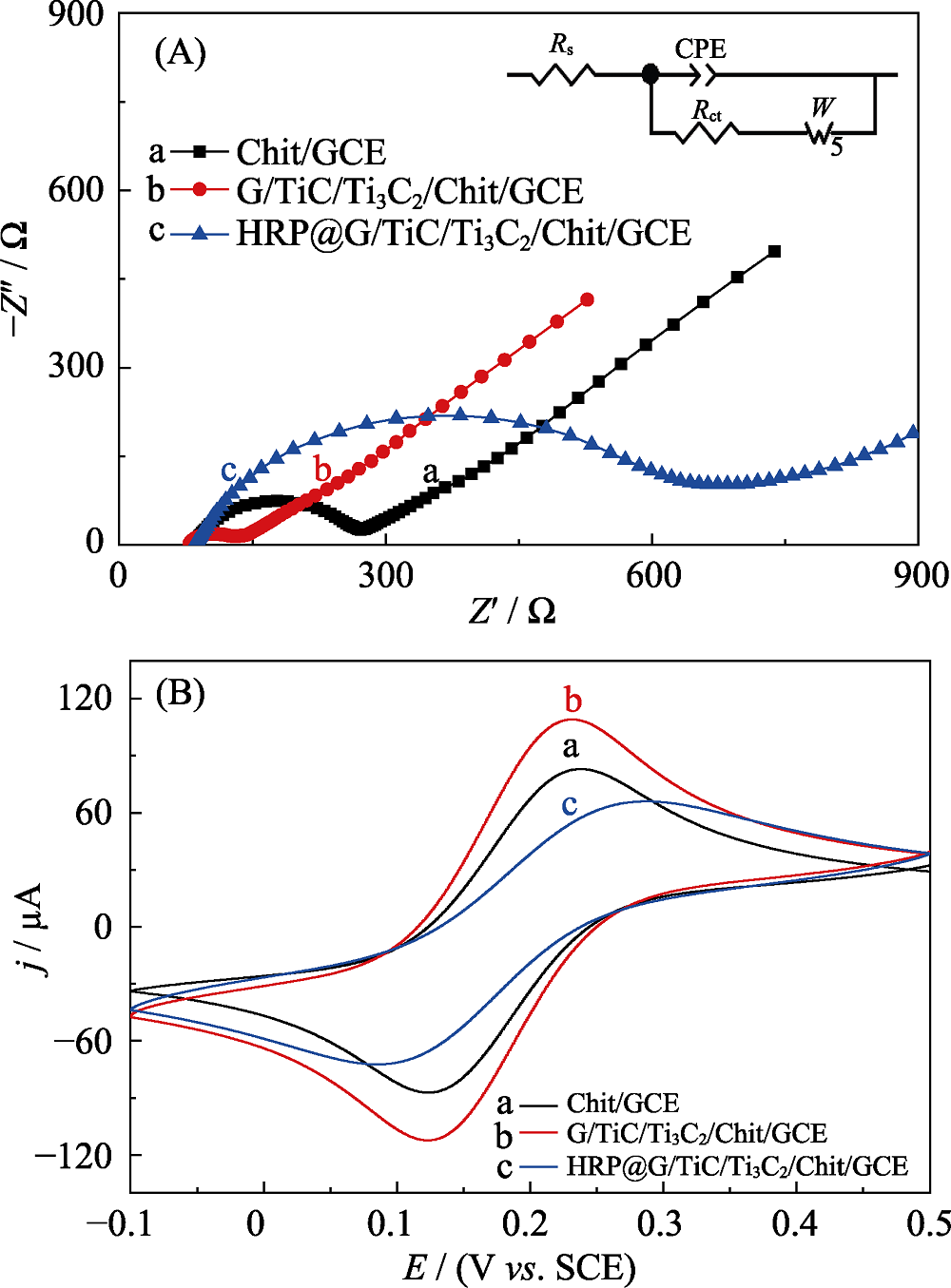
Fig. 3 EIS of Chit(chitosan)/GCE(a), MXene/Chit/GCE(b), HRP@MXene/Chit/GCE (c) electrodes cycled in 0.1 mol?L-1 KCL aqueous solution containing 5 mmol?L-1 [Fe(CN)6]3-/4- (A); CV curves of Chit/GCE (a), MXene/Chit/GCE (b), HRP@MXene/Chit/GCE (c) electrodes cycled in 0.1 mol?L-1 KCL aqueous solution containing 5 mmol?L-1 [Fe(CN)6]3-/4-: (potential window: -0.1-0.5 V vs. SCE) (B)
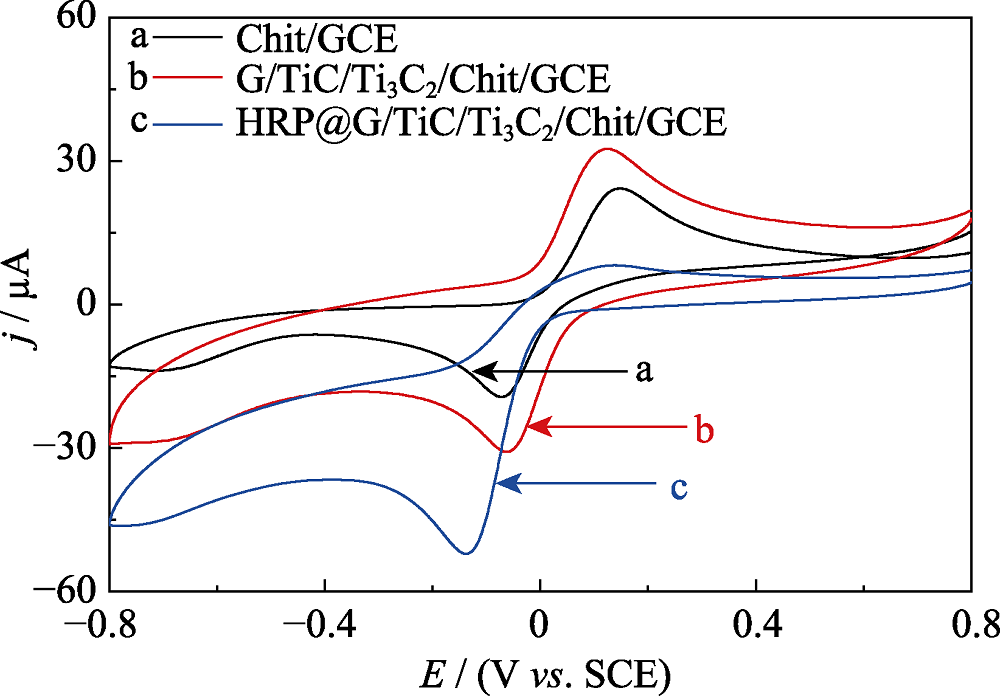
Fig. 4 CV curves of Chit/GCE (curve a, black line), MXene/ Chit/GCE (curve b, red line), HRP/Chit/GCE (curve c, pink line), HRP@MXene/Chit/GCE (curve d, blue line) electrodes cycled in N2-saturated 0.1 mol?L-1 PBS (pH 7.5) containing 1.0 mmol?L-1 HQ and 2.0 mmol?L-1 H2O2 at a scanning rate of 50 mV?s-1 (potential window: -0.8-0.8 V vs. SCE).
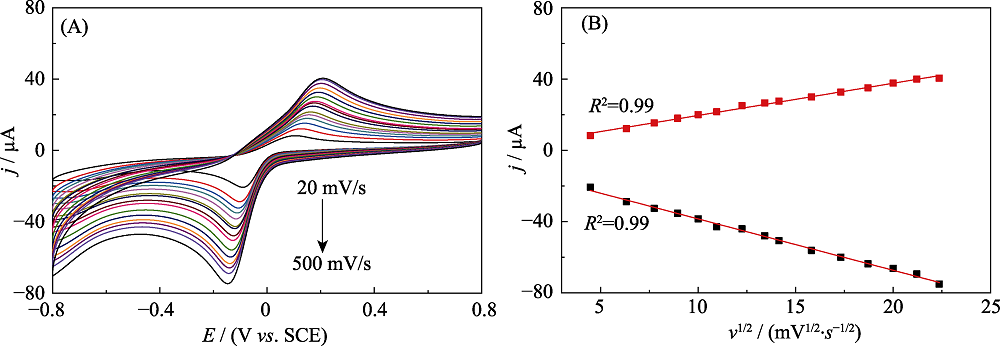
Fig. S2 CV curves of HRP@MXene/Chit/GCE electrodes cycled in N2-saturated 0.1 mol?L-1 PBS (pH 7.5) containing 1.0 mmol?L-1 HQ and 2.0 mmol?L-1 H2O2 at a different scanning rates (20-500 mV?s-1) (A); Plot of cathodic and anodic peak current for HRP@MXene/Chit/GCE versus scanning rate (B); Inset: Plots of anodic peak potential and cathodic peak potential for HRP@MXene/Chit/GCE electrode versus the logarithm of scan rate
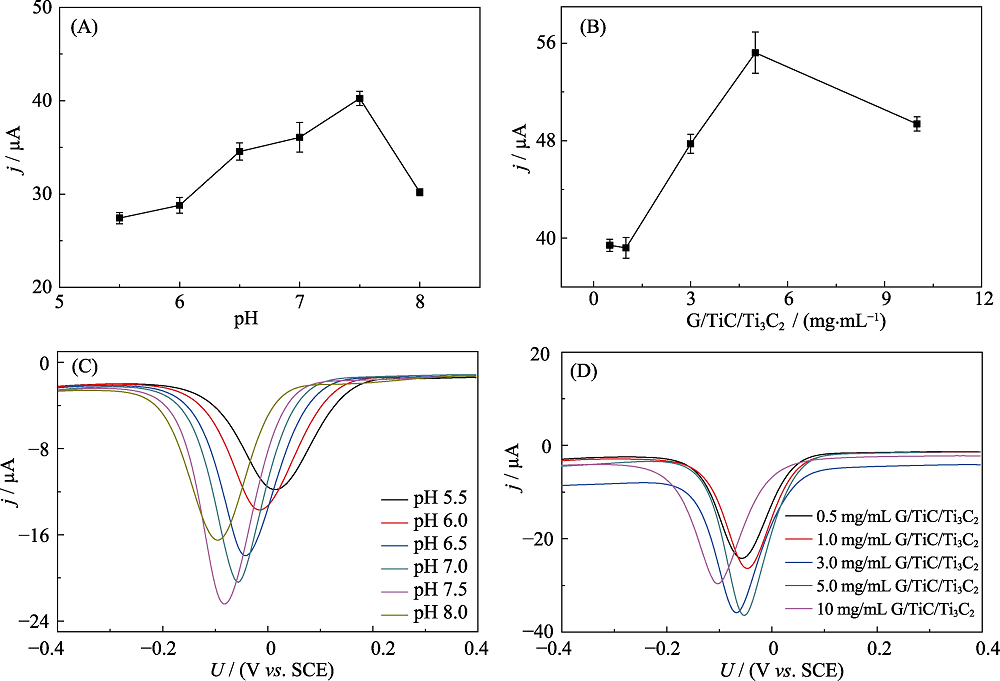
Fig.S3 Effects of PBS buffer’s pH (A) and concentration of MXene (B) on the cathodic peak current of enzyme biosensor cycled in N2-saturated 0.1 mol?L-1 PBS ( pH 7.5) containing 1.0 mmol?L-1 HQ and 2.0 mmol?L-1 H2O2; Effects of PBS buffer’s pH (C) and concentration of MXene (D) on the DPV response of enzyme biosensor cycledin N2-saturated 0.1 mol?L-1 PBS (pH 7.5) containing 1.0 mmol?L-1 HQ and 2.0 mmol?L-1 H2O2
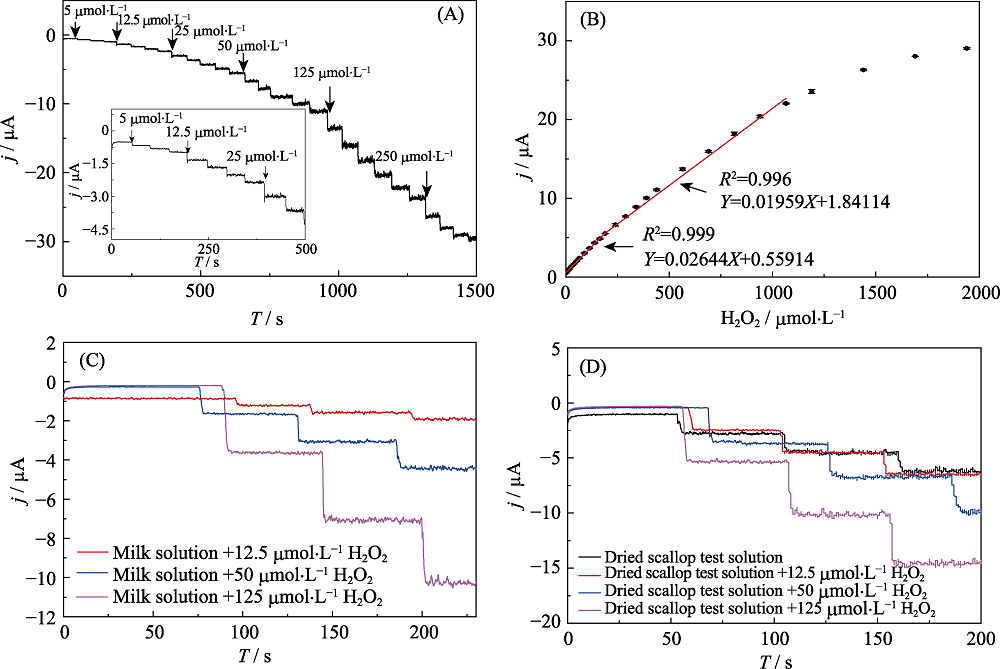
Fig. 5 Amperometric responses of HRP@MXene/Chit/ GCE at -0.1 V upon successive additions of H2O2 in astirred 0.1 mol?L-1 PBS (pH 7.5) (A); Calibration curve of amperometric responses at different H2O2 concentrations (B); Amperometric responses of HRP@MXene/Chit/ GCE at -0.1 V upon successive additions of solutions extracted from milk sample (C) and dried scallop (D) spiked with different H2O2 under stirred 0.1 mol?L-1 PBS (pH 7.5)
| Electrode | Linear range/(mmol?L-1) | LOD/(mmol?L-1) | Ref. |
|---|---|---|---|
| HRP-CTAB-Au/GCE | 0.50-105 | 0.23 | [1] |
| HRP/GO/GCE | 0.002-0.5 | 1.6 | [2] |
| HRP/TB/CCB | 0.429-455 | 0.17 | [3] |
| HRP-BMIM·BF4/SWCNTs | 0.49 to 10.2 | 0.13 | [4] |
| HRP/PGN/GCE | 2.77-835 | 2.67 ×10-4 | [5] |
| Hb-MXene-GO/Au foil | 2-1×103 | 1.95 | [6] |
| MXene/GCE | - | 0.7×10-3 | [7] |
| Hb-naf-MXene/GCE | 0.1-260 | 0.02 | [8] |
| TiO2-Hb-naf-MXene/GCE | 0.1-380 | 1.4×10-2 | [9] |
| HRP@MXene/Chitosan/GCE | 5-1.65×103 | 0.74 | This work |
Table S1 Comparison of the performance of present work with other published electrodes for hydrogen peroxide detection
| Electrode | Linear range/(mmol?L-1) | LOD/(mmol?L-1) | Ref. |
|---|---|---|---|
| HRP-CTAB-Au/GCE | 0.50-105 | 0.23 | [1] |
| HRP/GO/GCE | 0.002-0.5 | 1.6 | [2] |
| HRP/TB/CCB | 0.429-455 | 0.17 | [3] |
| HRP-BMIM·BF4/SWCNTs | 0.49 to 10.2 | 0.13 | [4] |
| HRP/PGN/GCE | 2.77-835 | 2.67 ×10-4 | [5] |
| Hb-MXene-GO/Au foil | 2-1×103 | 1.95 | [6] |
| MXene/GCE | - | 0.7×10-3 | [7] |
| Hb-naf-MXene/GCE | 0.1-260 | 0.02 | [8] |
| TiO2-Hb-naf-MXene/GCE | 0.1-380 | 1.4×10-2 | [9] |
| HRP@MXene/Chitosan/GCE | 5-1.65×103 | 0.74 | This work |
| Sample | Added H2O2/ (mmol?L-1) | Found H2O2/ (mmol?L-1) | Recovery /% | RSD /% |
|---|---|---|---|---|
| Milk | 12.5 | 13.037 | 104.30 | 5.88 |
| Milk | 50 | 52.57 | 105.14 | 1.12 |
| Milk | 125 | 136.5 | 109.20 | 3.33 |
| Dried scallop | 0 | 66.56 | - | - |
| Dried scallop | 12.5 | 77.84 | 90.24 | 6.97 |
| Dried scallop | 50 | 120.08 | 107.04 | 1.46 |
| Dried scallop | 125 | 189.11 | 98.04 | 8.39 |
Table 1 Detection of hydrogen peroxide in real food sample
| Sample | Added H2O2/ (mmol?L-1) | Found H2O2/ (mmol?L-1) | Recovery /% | RSD /% |
|---|---|---|---|---|
| Milk | 12.5 | 13.037 | 104.30 | 5.88 |
| Milk | 50 | 52.57 | 105.14 | 1.12 |
| Milk | 125 | 136.5 | 109.20 | 3.33 |
| Dried scallop | 0 | 66.56 | - | - |
| Dried scallop | 12.5 | 77.84 | 90.24 | 6.97 |
| Dried scallop | 50 | 120.08 | 107.04 | 1.46 |
| Dried scallop | 125 | 189.11 | 98.04 | 8.39 |
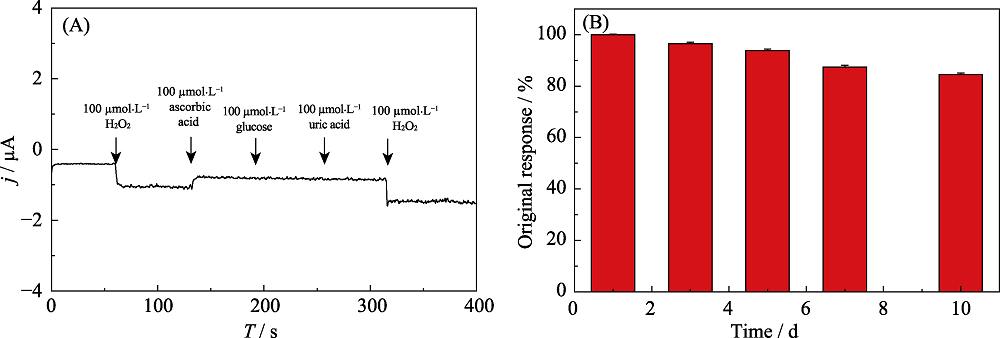
Fig.S4 Amperometric response of HRP@MXene/Chit/GCE in 0.1 mol?L-1 pH 7.5 PBS containing 100 mmol?L-1 of ascorbic acid, glucose, uric acid and H2O2 (Applied potential: -0.1 V) (A); Reduction peak currents of HRP@MXene/Chit/GCE stored in 50 mmol?L-1 PBS (pH 7.5) at 4 for 10 d (B)
| [1] |
ZHANG R, CHEN W . Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosensors & bioelectronics, 2017,89(Pt1):249-268.
DOI URL PMID |
| [2] | ADMINISTRATION F D . Code of Federal Regulations, 21CFR184.1366 2018. |
| [3] |
DAI H, LU W, ZUO X ,et al. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosensors & Bioelectronics, 2017,95:131-137.
DOI URL PMID |
| [4] | WANG Y, ZHAO K J, ZHANG Z Q ,et al. Simple approach to fabricate a highly sensitive H2O2 biosensor by one-step of graphene oxide and horseradish peroxidase co-immobilized glassy carbon electrode. International Journal of Electrochemical Science, 2018,13(3):2921-2933. |
| [5] |
CHANG M C Y, PRALLE A, ISACOFF E Y ,et al. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. Journal of the American Chemical Society, 2004,126(47):15392-15393.
DOI URL PMID |
| [6] |
SHARMA M, KOTHARI C, SHERIKAR O ,et al. Concurrent estimation of amlodipine besylate, hydrochlorothiazide and valsartan by RP-HPLC, HPTLC and UV-Spectrophotometry. Journal of Chromatographic Science, 2014,52(1):27-35.
DOI URL |
| [7] |
MATSUBARA C, KAWAMOTO N, TAKAMURA K . Oxo[5, 10, 15, 20-tetra(4-pyridyl)porphyrinato]titanium(IV): an ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst, 1992,117(11):1781-1784.
DOI URL |
| [8] |
ZHOU K, ZHU Y, YANG X ,et al. A novel hydrogen peroxide biosensor based on Au-graphene-HRP-chitosan biocomposites. Electrochimica Acta, 2010,55(9):3055-3060.
DOI URL |
| [9] |
THENMOZHI K, NARAYANAN S S . Horseradish peroxidase and toluidine blue covalently immobilized leak-free Sol-Gel composite biosensor for hydrogen peroxide. Materials Science & Engineering C,Materials for Biological Applications, 2017,70(Pt1):223-230.
DOI URL PMID |
| [10] |
MA B K, CHEONG L Z, WENG X C ,et al. Lipase@ZIF-8 nanoparticles-based biosensor for direct and sensitive detection of methyl parathion. Electrochimica Acta, 2018,283:509-516.
DOI URL |
| [11] |
JOS´E I, REYES-DE-CORCUERA H E O, GARC´ıA-TORRES A R . Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. 2018.
DOI URL PMID |
| [12] |
LIU Y, LIU X, GUO Z ,et al. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively.Biosensors & Bioelectronics, 2017,87:101-107.
DOI URL PMID |
| [13] |
ZHENG J, DIAO J, JIN Y ,et al. An inkjet printed Ti3C2-GO electrode for the electrochemical sensing of hydrogen peroxide. Journal of The Electrochemical Society, 2018,165(5):B227-B231.
DOI URL PMID |
| [14] |
ZHAO M Q, XIE X, REN C E ,et al. Hollow mxene spheres and 3D macroporous mxene frameworks for Na-ion storage. Advanced Materials, 2017,29(37):1702410.
DOI URL PMID |
| [15] |
ZHOU J, ZHA X, ZHOU X ,et al. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano, 2017,11(4):3841-3850.
DOI URL PMID |
| [16] |
XU B, ZHU M, ZHANG W ,et al. Ultrathin MXene-micropattern- based field-effect transistor for probing neural activity. Advanced Materials, 2016,28(17):3333-3339.
DOI URL PMID |
| [17] |
LORENCOVA L, BERTOK T, DOSEKOVA E ,et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: towards ultrasensitive H2O2 sensing. Electrochimica Acta, 2017,235:471-479.
DOI URL PMID |
| [18] |
LORENCOVA L, BERTOK T, FILIP J ,et al. Highly stable Ti3C2Tx(MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications. Sensors and Actuators B: Chemical, 2018,263:360-368.
DOI URL |
| [19] |
WANG F, YANG C, DUAN M ,et al. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosensors and Bioelectronics, 2015,74:1022-1028.
DOI URL PMID |
| [20] |
LIU H, DUAN C, YANG C ,et al. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sensors and Actuators B: Chemical, 2015,218:60-66.
DOI URL PMID |
| [21] |
RAKHI R B, NAYAK P, XIA C ,et al. Novel amperometric glucose biosensor based on MXene nanocomposite. Scientific Reports, 2016,6:36422.
DOI URL PMID |
| [22] |
VEITCH N C . Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry, 2004,65(3):249-259.
DOI URL PMID |
| [23] |
REN Q Q, WU J, ZHANG W C ,et al. Real-time in vitro detection of cellular H2O2 under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. Sensors and Actuators B: Chemical, 2017,245:615-621.
DOI URL |
| [24] |
LI M, HAN M, ZHOU J ,et al. Novel scale-like structures of graphite/TiC/Ti3/C2 hybrids for electromagnetic absorption.Advanced Electronic Materials, 2018,4(5):1700617.
DOI URL |
| [25] |
SHAN C, YANG H, HAN D ,et al. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosensors & bioElectronics, 2010,25(5):1070-1074.
DOI URL PMID |
| [26] |
WANG F, YANG C, DUAN C ,et al. An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor. Journal of The Electrochemical Society, 2014,162(1):B16-B21.
DOI URL |
| [27] |
KANG X B, PANG G C, LIANG X Y ,et al. Study on a hydrogen peroxide biosensor based on horseradish peroxidase/GNPs-thionine/ chitosan. Electrochimica Acta, 2012,62:327-334.
DOI URL |
| [28] |
KOPOSOVA E, LIU X, KISNER A ,et al. Bioelectrochemical systems with oleylamine-stabilized gold nanostructures and horseradish peroxidase for hydrogen peroxide sensor. Biosensors & Bioelectronics, 2014,57:54-58.
DOI URL PMID |
| [29] |
YANG S, DING S, LI L ,et al. One-step preparation of direct electrochemistry HRP biosensor via electrodeposition. Journal of The Electrochemical Society, 2017,164(13):B710-B714.
DOI URL |
| [30] |
CHEN W, YANG W, LU Y ,et al. Encapsulation of enzyme into mesoporous cages of metal-organic frameworks for the development of highly stable electrochemical biosensors. Analytical Methods, 2017,9(21):3213-3220.
DOI URL |
| [31] |
BARD A J, FAULKNER L R, LEDDY J , et al. Electrochemical methods: Fundamentals and Applications. Wiley New York, 1980.
DOI URL PMID |
| [32] |
SONG H, NI Y, KOKOT S . Investigations of an electrochemical platform based on the layered MoS2-graphene and horseradish peroxidase nanocomposite for direct electrochemistry and electrocatalysis. Biosensors & Bioelectronics, 2014,56:137-143.
DOI URL PMID |
| [33] |
MART N M, SALAZAR P, VILLALONGA R ,et al. Preparation of core-shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction. J. Mater. Chem. B, 2014,2(6):739-746.
DOI URL |
| [1] | 何倩, 唐婉兰, 韩秉锟, 魏佳元, 吕文轩, 唐昭敏. pH响应铜掺杂介孔硅纳米催化剂增强肿瘤化疗-化学动力学联合治疗的研究[J]. 无机材料学报, 2024, 39(1): 90-98. |
| [2] | 刘瑶, 尤勋海, 赵冰, 罗晓莹, 陈星. 功能纳米材料应用于电化学新冠病毒生物传感器的研究进展[J]. 无机材料学报, 2023, 38(1): 32-42. |
| [3] | 李妍妍, 彭宇思, 林成龙, 罗晓莹, 滕峥, 张曦, 黄政仁, 杨勇. 用于新型冠状病毒检测的纳米材料及生物传感技术[J]. 无机材料学报, 2023, 38(1): 3-31. |
| [4] | 陈小梅, 陈颖, 袁霞. 核壳材料Co3O4@SiO2催化环己基过氧化氢分解[J]. 无机材料学报, 2022, 37(1): 65-71. |
| [5] | 郭灵霞, 施雨辰, 赵振杰, 李欣. 微流控技术制备ZnO纳米棒及生物荧光检测性能研究[J]. 无机材料学报, 2018, 33(10): 1103-1109. |
| [6] | 郭露露, 李立霞, 何鹏程, 袁 霞. 介孔材料Co/SBA-15催化环己基过氧化氢分解的研究[J]. 无机材料学报, 2017, 32(5): 543-549. |
| [7] | 赵得瑞, 翟英娇, 李金华, 楚学影, 徐铭泽,李 雪, 方 铉, 魏志鹏, 王晓华. 基于花状MoS2微米材料的葡萄糖生物传感器的制备及其性能研究[J]. 无机材料学报, 2016, 31(2): 153-158. |
| [8] | 卢书培, 冯利利, 齐 麟, 王丽丽, 齐兴义. Buserite型氧化锰催化叔丁基过氧化氢歧化分解反应动力学[J]. 无机材料学报, 2016, 31(1): 14-20. |
| [9] | 章俞之,王忠春,快素兰,胡行方. 锂掺杂MoO3薄膜的制备及电色性能的研究[J]. 无机材料学报, 2000, 15(6): 1131-1135. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||