In recent years, with the increasing demand for spallation neutron sources, particle therapy, space effect irradiation, and other devices, the use of synchrotron has never been more extensive, which results in the continuous demand for high-grade soft magnetic alloys[1]. The optimized goal of the soft magnetic alloy includes mainly reducing energy losses and increasing saturation inductions at high operating frequencies[2,3].
The energy loss of soft magnetic alloys consists of hysteresis loss, eddy current loss, and excess loss. Under the high frequency operating current, the eddy current loss dominates[4], which can be effectively reduced by applying an insulating coating on the surface of the soft magnetic alloy. The hysteresis loss arises from the internal residual stress caused by the increased density of defects and dislocations generated in the cold compaction process of the soft magnetic alloys. The subsequent heat-treatment (above 500 ℃) is an effective method to release residual stress by reducing imperfections, which results in a reduction in hysteresis loss. Therefore, the high-temperature resistance and insulating coating can significantly improve the soft magnetic alloy's performance.
Normally, the organic coatings, such as silicone and phenolic resins, have thermal resistance below 200 ℃, while the inorganic coating, such as Al2O3[5,6], SiO2[7,8,9], TiO2[10], offers an advantage of tolerating high temperature (above 500 ℃). However, most of the reports are about the preparation of insulation on the surface of the powder, which remains a need for an efficient method to prepare the insulating films on the surface of the soft magnetic alloy. SiO2 exhibits excellent insulation properties due to its large bandgap of 8.9 eV and dielectric constant of 3.9. There are many methods for preparing SiO2 coating, such as the self-assembly method, Sol-Gel method, spraying method, impregnation method, brush coating method, and so on. And the use of the Sol-Gel method to prepare SiO2 coating on the surface of soft magnetic alloy has advantages including: 1) low synthesis temperature; 2) convenience for large-area preparation; 3) uniform and controllable thickness; 4) low raw material costs; 5) simple preparation process.
When small molecular acids such as hydrochloric acid and sulfuric acid are used as catalysts, the film-forming property of SiO2 coating is not good, due to the weak bonding force between the SiO2 coating and the substrate. The phytic acid (C6H18O24P6), a natural and innoxious organic big molecule compound, consists of 24 oxygen atoms, 12 hydroxyl groups, and 6 phosphate groups, which makes phytic acid have powerful chelating capability with many metal ions[11].
In this work, the phytic acid is used as a catalyst to prepare a silicon sol, and different amounts of KH-560 are used as a silicone coupled agent to modify the SiO2 coating on the surface of the soft magnetic alloy via the Sol-Gel method. The microstructure and electrical properties of the SiO2 insulating coating were investigated in detail. The coating has good high-temperature resistance and excellent insulation performance, which can be used on the surface of the soft magnetic alloy.
1 Experimental
1.1 Materials and chemical reagents
The MTES, KH-560, TEOS, and 70% (weight percent) aquous phytic acid solution were purchased from the Aladdin Reagent Co., Ltd. (China).
1.2 Preparation of SiO2 coating
In a typical process, different additions of KH-560 (0.01-0.04 mol), 0.0012 mol of phytic acid, and 0.4 mol of ethanol were poured into a 150 mL beaker and reacted at 70 ℃ for 1 h under magnetic stirring. Then, 0.15 mol of MTES, 0.03 mol of TEOS, 0.6 mol of deionized water, and 0.2 mol of ethanol were poured into the above- mentioned beaker. Finally, the mixture was continued to react at 70 ℃ for 1 h to obtain the silicon sol.
The SiO2 coating was prepared by dip-coating method. In a typical process, the soft magnetic alloy was dipped into silicon sol for 1 min at a constant rate of 4×103 mm/s. Then, the coatings were air-dried at room temperature for 12 h. The soft magnetic alloy with SiO2 coating was thermal treatment at 570 ℃ for 80 min under a nitrogen atmosphere. The obtained different coating was named SiO2-0.01 KH, SiO2-0.02 KH, SiO2-0.03 KH, SiO2-0.04 KH, respectively.
1.3 Characterizations
Phase identification of the soft magnetic alloys before and after the coating process was performed using X-ray diffraction (XRD, Rigaku MiniFlex600X, Japan) with Cu Kα radiation. Microstructures and element composition of the SiO2 coating were measured through S3400 scanning electron microscope (SEM) and energy dispersive spectroscopy (EDS) with 15 kV acceleration voltages. Fourier transforms infrared (FT-TR) spectra were recorded by spectrophotometer (Magna-IR 560, Nicolet Co.) in the spectral range 500-4000 cm-1 to investigate the composition of the insulating coating. The sheet resistance was measured by using a high resistance analyzer MCP-HT800. The electrochemical measurements were implemented by the Solartron SI1287 Advanced Electrochemical Interface instrument & Solartron SI1260 Impedance/Gain-Phase Analyzer.
2 Results and discussions
2.1 Characterization and chemical composition
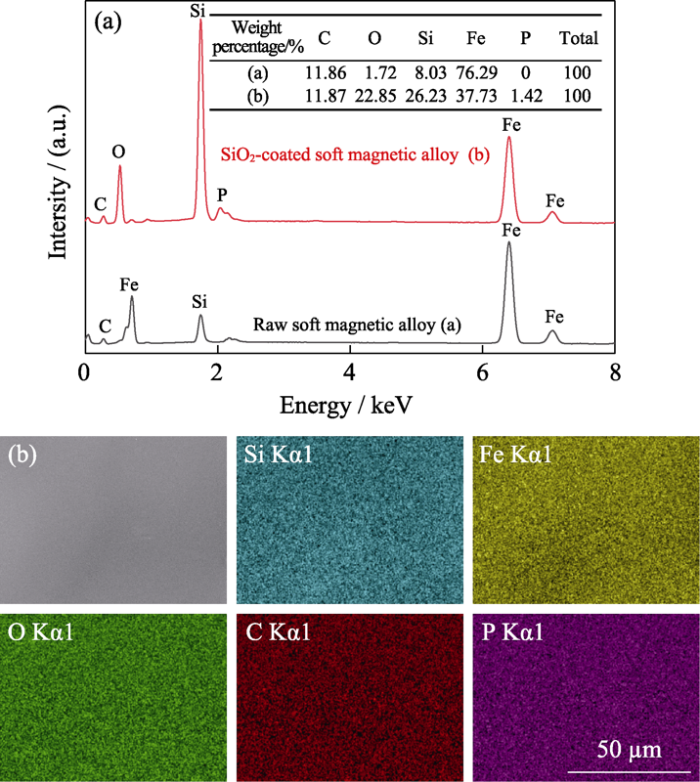
The chemical composition of the coating was elucidated through the EDS method. Fig. 1 shows the elemental compositions of the soft magnetic alloy before and after the coating process. In Fig. 1(a), the soft magnetic alloy coated with SiO2 coating contains the C, O, P, and Si elements. The P element comes from phytic acid. Besides, the EDS profiles indicated that the O and Si signals on the soft magnetic alloy surface were enhanced after coated with SiO2 coating compared to the raw soft magnetic alloy. The mass fraction of the O element increases from 1.72% to 22.85%, and that of the Si element increases from 8.03% to 26.23%, respectively. These results imply that the coating is SiO2. In Fig. 1(b), it is observed that C, O, Si, and P atoms are uniformly distributed on the surface of the soft magnetic alloy.
Fig. 1
Fig. 1
(a) EDS spectra of the soft magnetic alloy before and after the coating process, and (b) EDS mapping of SiO2 coating on the soft magnetic alloy
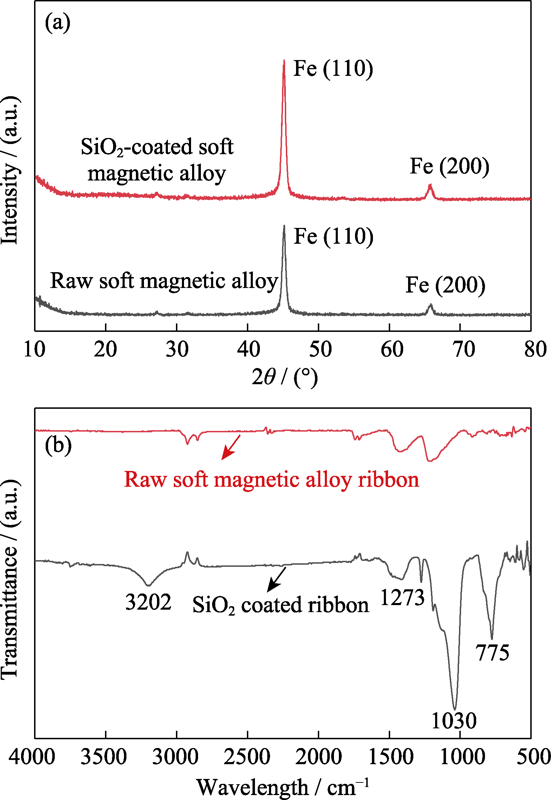
Fig. 2(a) displays the XRD patterns of the soft magnetic alloy before and after the coating process. The diffraction peak of Fe(110) at around 2θ=45° can be observed, and the XRD patterns of the soft magnetic alloy before and after the chemical coating process are similar. Based on the XRD patterns and EDS data, it can be confirmed that an amorphous SiO2 phase is formed on the surface of the soft magnetic alloy.
Fig. 2
Fig. 2
(a) XRD patterns of the soft magnetic alloy before and after coating process, and (b) FT-IR spectra of the soft magnetic alloy before and after coating process
To further confirm the presence of SiO2 coating on the soft magnetic alloy, the FT-IR spectra of the soft magnetic alloy before and after the coating process were measured. As shown in Fig. 2(b), compared with the soft magnetic alloy, some characteristic absorption peaks are observed after coating. The peak at around 1030 cm-1 is attributed to the asymmetric stretching vibration of Si-O-Si, and the symmetric stretching vibration of Si-O-Si gives rise to the peak at around 775 cm-1[12]. The peak with the center located at around 1273 cm-1 was associated with the stretching vibration of Si-CH3 groups. Moreover, the peak with the center located at around 3202 cm-1 was associated with the stretching vibration of the -OH bond, which can be contributed to the excessive amounts of the hydroxyl groups during the hydrolysis and condensation processes[5]. Therefore, the appearances of those characteristic peaks further verify that the obtained coating on the surface of the soft magnetic alloy is SiO2 coating.
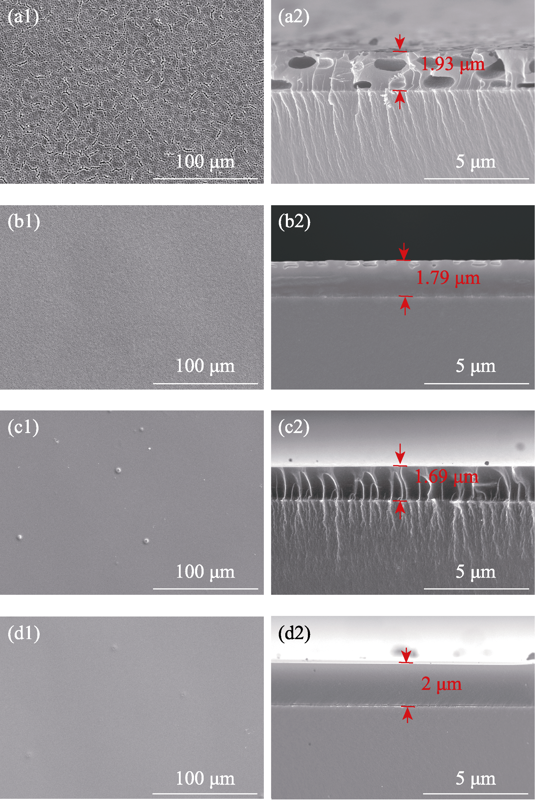
The microstructures of the different SiO2 coating are shown in Fig. 3. As shown in Fig. 3(a1), the SiO2-0.01 KH coating contains some obvious cracks, which may be formed because of the higher inner stress. It could not completely cover the soft magnetic alloy surface. In Fig. 3(a2), the thickness of SiO2-0.01 KH coating is about 1.93 μm, and there are several holes in the coating section. With the increase of KH-560, the alloy surface is covered by a complete coating as shown in Fig. 3(b1, b2). But the SiO2-0.02 KH coating still has some defects as shown in the coating section. For the SiO2-0.03 KH coating and SiO2-0.04 KH coating, their surfaces are more smooth and uniform. The SiO2-0.04 KH coating possesses the most complete section and uniform thickness as shown in Fig. 3(d2).
Fig. 3
Fig. 3
SEM images of (a) SiO2-0.01 KH, (b) SiO2-0.02 KH, (c) SiO2-0.03 KH, and (d) SiO2-0.04 KH
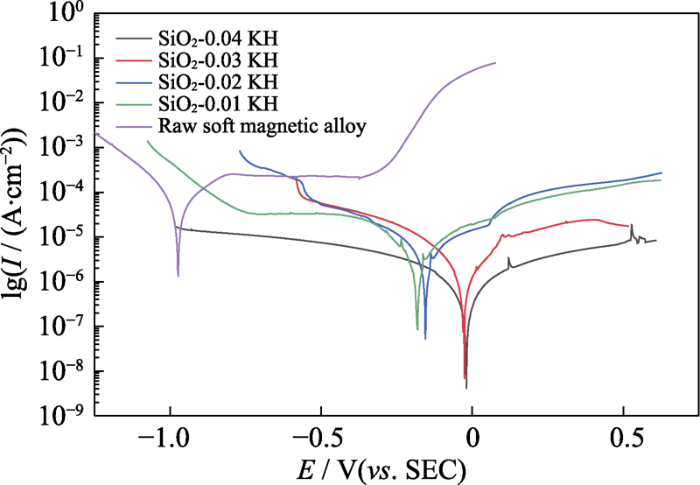
Tafel polarization curves can characterize the corrosion resistance. Fig. 4 shows the Tafel polarization curves of SiO2 coating with different adding amounts of KH-560. Compared with the soft magnetic alloy without coating, the corrosion current density decreases after coating, and the corrosion potential of different SiO2-xKH samples shifts to the positive electrode region, which indicates that the corrosion resistance of the soft magnetic alloy is improved obviously after coating. The reason is that SiO2 coating on the surface of the soft magnetic alloy slows down the corrosion rate.
Fig. 4
Fig. 4
Tafel polarization curves of the soft magnetic alloy with different SiO2 coating
In Table 1, the corrosion potential (Ecorr), corrosion current (Icorr), and polarization resistance (Rp) are obtained by the Tafel epitaxial method. The corrosion current of the raw soft magnetic alloy is 4.0982×10-5 A/cm2. Compared with the performance of 4 coating samples, the SiO2-0.04 KH coating has the lowest corrosion current of 2.9071×10-7 A/cm2 and the highest polarization resistance of 76910 Ω·cm2. The corrosion current of SiO2-0.04 KH coating is less than 1/10 of that of SiO2-0.01 KH coating. The protective efficiency (PE) of the coating is calculated as follows:
where, I0c and Ic are the corrosion current without coating and the corrosion current of SiO2 coating, respectively. From Table 1, the protection efficiency of SiO2-0.01 KH coating is 81.95%, and that is up to 99.29% of SiO2-0.04 KH coating. This indicates that the SiO2-0.04 KH coating has the best corrosion resistance due to its good film-forming properties.
Table 1 Sheet resistance of different SiO2 coating
| Sample | Ecorr/V (vs. SEC) | Icorr/(A·cm-2) | Rp/(Ω·cm2) | PE/% |
|---|---|---|---|---|
| Raw soft magnetic alloy | -0.973 | 4.0982×10-5 | 552.31 | - |
| SiO2-0.01 KH | -0.182 | 7.398×10-6 | 8559.9 | 81.95 |
| SiO2-0.02 KH | -0.150 | 3.4603×10-7 | 6519.6 | 99.16 |
| SiO2-0.03 KH | -0.065 | 3.1833×10-7 | 22077 | 99.22 |
| SiO2-0.04 KH | -0.007 | 2.9071×10-7 | 76910 | 99.29 |
2.2 Mechanism analysis
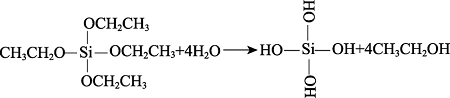
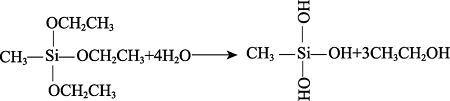
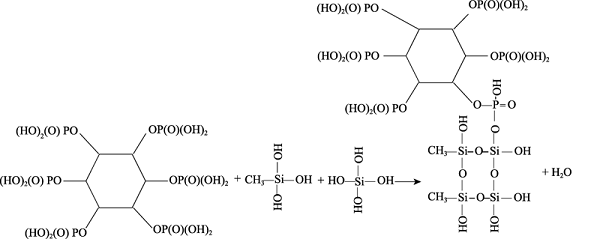
Formula (2) and (3) show the hydrolysis reactions of MTES and TEOS, which produces ethanol, Si(OH)4, or CH3Si(OH)3. As shown in formula (4), the P-OH groups of phytic acid react with the Si-OH group to form the Si-O-P bond that binds the phytic acid molecules with the silicon-oxygen chain. Besides, in formula (5), the P-OH in phytic acid and the epoxy bond of KH-560 can also bond in the ring-opening reaction of epoxy, which further improves the degree of cross-linking between phytic acid and Si-O-Si, and increase the P-OH groups in silica sol. Then, the product continues to undergo co-hydrolysis and condensation reactions with MTES and TEOS to form the phytic acid-bound siloxane network structure[11].
Usually, the phosphate group, carboxyl group, and other functional groups are more easily absorbed on oxide metal surfaces. When the soft magnetic alloy is immersed in the SiO2 solution, the surface can be slightly oxidized by the phytic acid containing 6 phosphate groups that promote adsorption of the SiO2 molecule[13,14,15,16]. In addition, the remaining P-OH groups in phytic acid can provide a strong chemical interaction between the substrate and the coating to improve the adhesion between them[17].
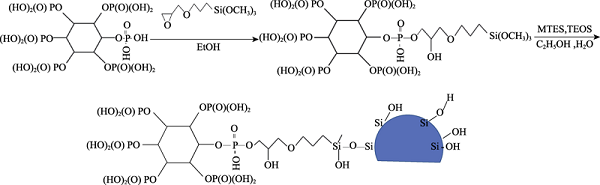
As shown in formula (5), the 3-glycidyl ether oxypropyl group from coupling agent KH-560 are much longer than the methyl group of MTES. These long-chain organic groups were introduced into the inorganic skeleton of Si-O-Si via the addition of KH-560, which further improves the flexibility of the SiO2 coating, enhances the ability of the film to resist stress, and reduces the generation of cracks.
2.3 Electrical resistance
The test results of sheet resistance of different SiO2 coating are shown in Table 2. When the test voltage is 10 V, the sheet resistance of SiO2-0.01 KH is only 109 Ω/□, while the sheet resistance of SiO2-0.02 KH, SiO2-0.03 KH, SiO2-0.04 KH is above 1010 Ω/□. When voltage increases to 50 and 100 V, the sheet resistance of SiO2-0.02 KH, SiO2-0.03 KH, SiO2-0.04 KH does not reduce obviously, except that of SiO2-0.01 KH. In particular, the SiO2-0.04 KH sample shows the best resistance characteristics. This is because KH-560 improves the stability and film-forming property of silicone[18]. When the addition of KH-560 increases from 0.01 mol to 0.04 mol, the sheet resistance increases significantly, and the SiO2-0.04 KH shows the best insulating property of 2.95×1011 Ω/□ at 100 V.
Table 2 Sheet resistance of different SiO2 coating at different voltages
| Sample | Sheet resistance at 10 V/(Ω/□) | Sheet resistance at 50 V/(Ω/□) | Sheet resistance at 100 V/(Ω/□) | |
|---|---|---|---|---|
| SiO2-0.01 KH | 1 | 2.33×109 | 3.66×108 | 2.92×108 |
| 2 | 8.68×108 | 4.58×108 | 7.56×108 | |
| 3 | 1.59×109 | 5.85×108 | 2.61×108 | |
| Average | 1.60×109 | 4.70×108 | 4.36×108 | |
| SiO2-0.02 KH | 1 | 1.82×1010 | 4.92×1010 | 8.88×109 |
| 2 | 4.55×1010 | 3.70×1010 | 4.40×109 | |
| 3 | 2.19×1010 | 2.71×1011 | 1.71×1011 | |
| Average | 2.85×1010 | 1.19×1011 | 6.14×1010 | |
| SiO2-0.03 KH | 1 | 8.71×1010 | 1.25×109 | 2.21×109 |
| 2 | 4.17×1010 | 6.55×1010 | 6.52×109 | |
| 3 | 5.68×1010 | 8.58×109 | 6.02×109 | |
| Average | 6.19×1010 | 2.51×1010 | 4.92×109 | |
SiO2-0.04 KH | 1 | 8.62×1010 | 2.81×1011 | 4.51×1010 |
| 2 | 3.33×1011 | 4.52×1010 | 2.76×1011 | |
| 3 | 1.91×1011 | 1.56×1010 | 5.70×1011 | |
| Average | 2.03×1011 | 1.14×1011 | 2.97×1011 |
3 Conclusions
The SiO2 coating was fabricated on the soft magnetic alloy surface through a dip-coating process. The mechanism of KH-560 improving the SiO2 coating performance was systematically analyzed. The reasonable KH-560 addition can enhance the stability and film-forming property of SiO2 coating. When the addition amount of KH-560 is 0.04 mol, the protection efficiency of as-prepared SiO2 coating is up to 99.29%, which exhibits the smoothest surface and the highest electrical insulation property of 2.97×1011 Ω/□ at 100 V compared with other samples. This coating has application potential on the soft magnetic alloy of proton/heavy ion synchrotron.
参考文献
Soft magnetic materials in high-frequency, high-power conversion applications
Soft magnetic composites: recent advancements in the technology
Soft magnetic composite materials (SMCs)
Surface oxidation and crystallization of FeNi-based soft magnetic nanocrystalline and amorphous nanocomposite alloys
Intergranular insulating reduced iron powder-carbonyl iron powder/SiO2-Al2O3 soft magnetic composites with high saturation magnetic flux density and low core loss
Effect of heat treatment on magnetic properties of iron-based soft magnetic composites with Al2O3 insulation coating produced by Sol-Gel method
Industry-oriented Fe-based amorphous soft magnetic composites with SiO2-coated layer by one-pot high-efficient synthesis method
Intergranular insulated Fe/SiO2 soft magnetic composite for decreased core loss
High permeability and low loss bioinspired soft magnetic composites with nacre-like structure for high frequency applications
Enhanced soft magnetic properties of the Fe-based amorphous powder cores with novel TiO2 insulation coating layer
The microstructure and anticorrosion performance of phytic acid-catalyzed polysilsesquioxane coatings
Reactivity characteristics of SiO2-coated zero-valent iron nanoparticles for 2,4-dichlorophenol degradation
Protection of passivated iron against corrosion in a 0.1 mol/L NaNO3 solution by coverage with an ultrathin polymer coating of carboxylate SAM
Surface modification of ZnO (0001)-Zn with phosphonate-based self-assembled monolayers: binding modes, orientation, and work function
Excellent anti-corrosive pretreatment layer on iron substrate based on three-dimensional porous phytic acid/silane hybrid
Preparation of self assembled sodium oleate monolayer on mild steel and its corrosion inhibition behavior in saline water
Effect of anti-corrosive performance, roughness and chemical composition of pre-treatment layer on the overall performance of the paint system on cold-rolled steel