
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (4): 440-448.DOI: 10.15541/jim20240222
Special Issue: 【能源环境】污染物催化去除(202506)
• RESEARCH LETTER • Previous Articles
LI Jianjun1,2,3( ), CHEN Fangming1, ZHANG Lili3, WANG Lei1, ZHANG Liting2,3, CHEN Huiwen1, XUE Changguo1, XU Liangji1,2
), CHEN Fangming1, ZHANG Lili3, WANG Lei1, ZHANG Liting2,3, CHEN Huiwen1, XUE Changguo1, XU Liangji1,2
Received:2024-04-28
Revised:2024-07-30
Published:2025-04-20
Online:2024-08-19
Contact:
LI Jianjun, professor. E-mail: ljj.hero@126.comAbout author:LI Jianjun (1975-), professor. E-mail: ljj.hero@126.com
Supported by:CLC Number:
LI Jianjun, CHEN Fangming, ZHANG Lili, WANG Lei, ZHANG Liting, CHEN Huiwen, XUE Changguo, XU Liangji. Peroxymonosulfate Activation by CoFe2O4/MgAl-LDH Catalyst for the Boosted Degradation of Antibiotic[J]. Journal of Inorganic Materials, 2025, 40(4): 440-448.

Fig. 2 SEM images of MgAl-LDH and CoFe2O4/MgAl-LDH, and EDS mappings of CoFe2O4/MgAl-LDH (a, b) SEM images of MgAl-LDH; (c, d) SEM images of CoFe2O4/ MgAl-LDH; (e) EDS scanning area; (f-j) EDS mappings of CoFe2O4/ MgAl-LDH
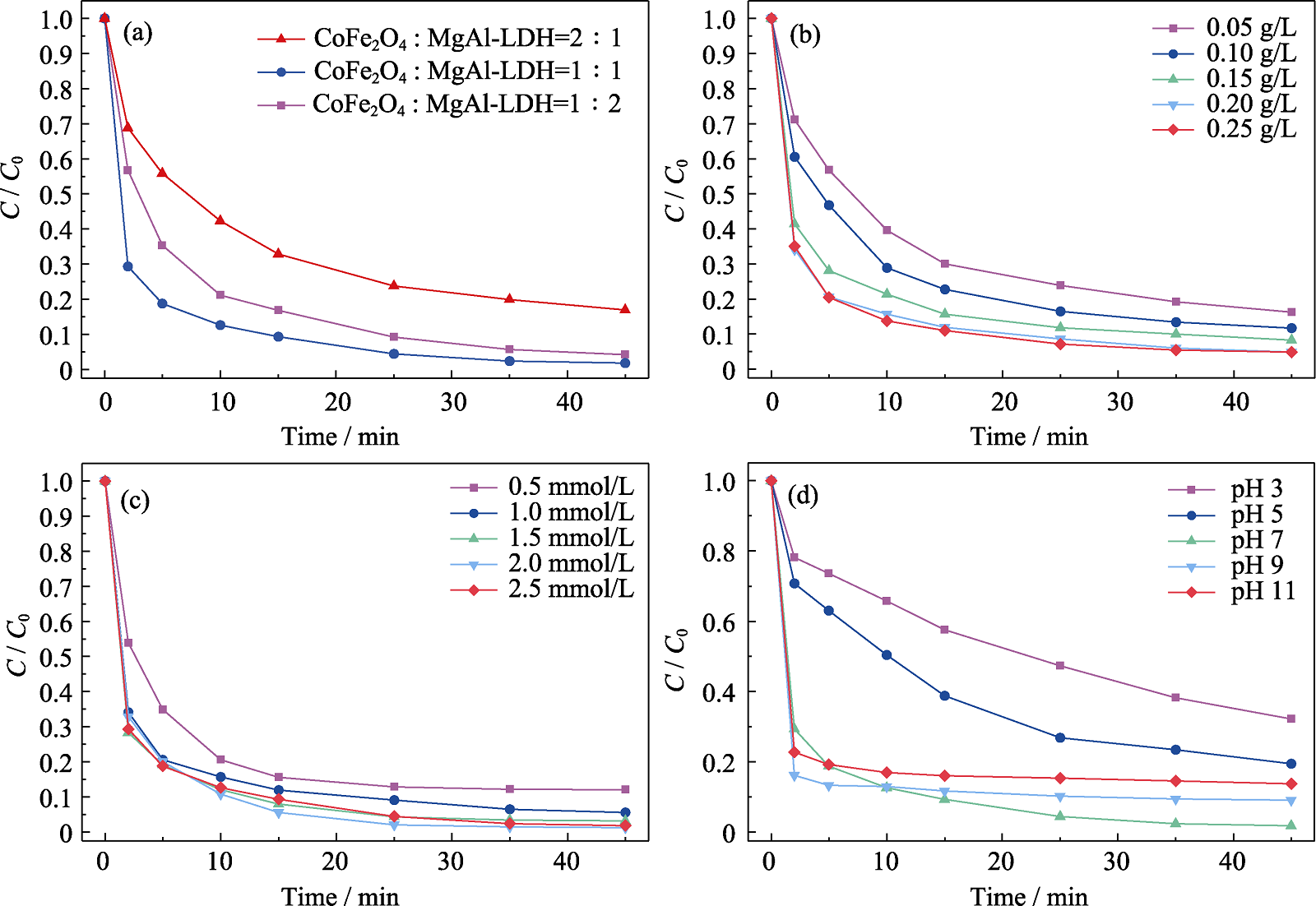
Fig. 6 Effects of different factors on TCH removal (a) Mass ratio of CoFe2O4 to MgAl-LDH; (b) Catalyst dosage; (c) PMS concentration; (d) pH ([TCH]=25 mg/L, [PMS]=1.5 mmol/L, CoFe2O4/MgAl-LDH=0.20 g/L, pH 7 and T=25 ℃)
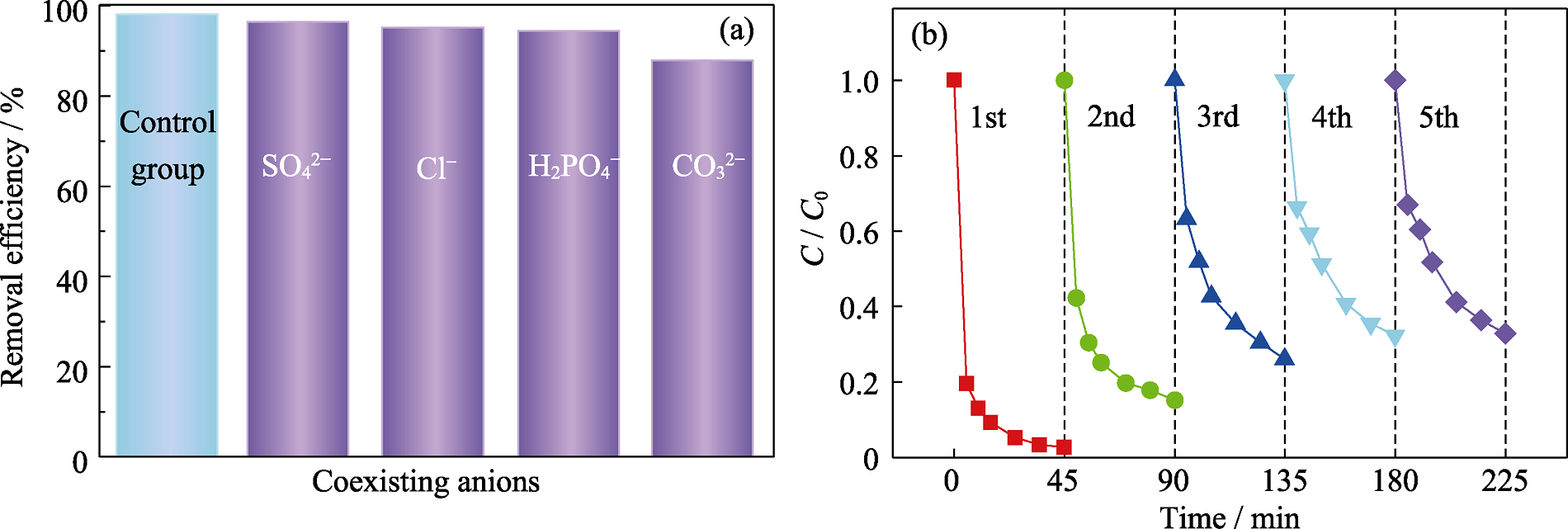
Fig. 7 Effects of coexisting anions on TCH removal (a) and cyclic experiments (b) ([TCH]=25 mg/L, [PMS]=1.5 mmol/L, CoFe2O4/MgAl-LDH=0.20 g/L, pH 7 and T=25 ℃)
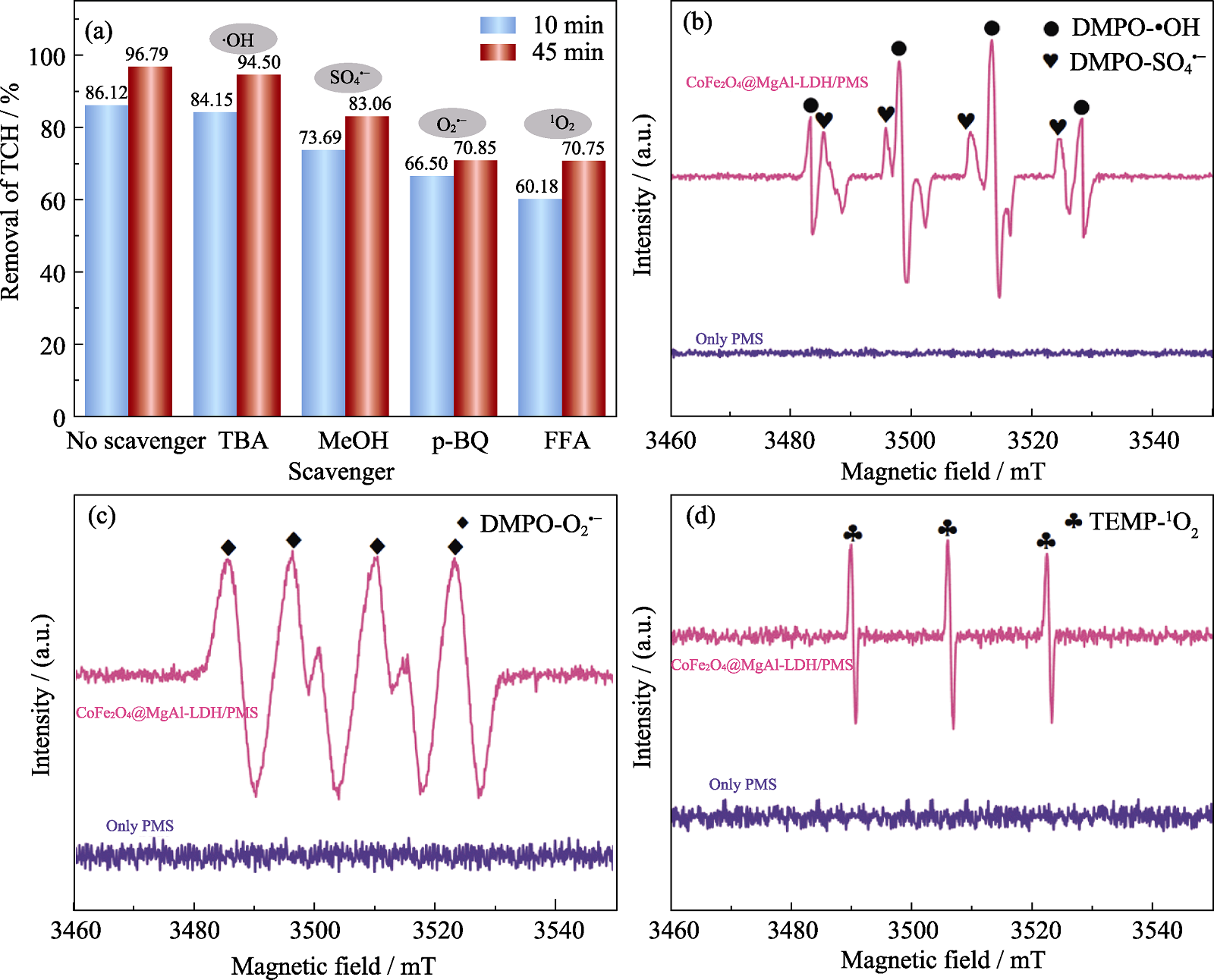
Fig. 8 Effects of ROS quenching tests on TCH removal (a) and EPR spectra of (b) DMPO-•OH and DMPO-SO4•−, (c) DMPO-O2•−, and (d) TEMP-1O2 ([TCH]=25 mg/L, [PMS]=1.5 mmol/L, CoFe2O4/MgAl-LDH=0.20 g/L, pH 7, T=25 ℃ and [Scavenger]=100 mmol/L)
| Sample | SBET/(m2·g-1) | Pore volume/ (cm3·g-1) | Pore size/nm |
|---|---|---|---|
| CoFe2O4 | 35.42 | 0.15 | 10.76 |
| MgAl-LDH | 138.78 | 0.16 | 5.76 |
| CoFe2O4/MgAl-LDH | 82.84 | 0.25 | 23.26 |
Table S1 SBET and pore size analysis data of the prepared CoFe2O4, MgAl-LDH and CoFe2O4/MgAl-LDH
| Sample | SBET/(m2·g-1) | Pore volume/ (cm3·g-1) | Pore size/nm |
|---|---|---|---|
| CoFe2O4 | 35.42 | 0.15 | 10.76 |
| MgAl-LDH | 138.78 | 0.16 | 5.76 |
| CoFe2O4/MgAl-LDH | 82.84 | 0.25 | 23.26 |
| Catalyst | Tetracycline concentration/(mg·L-1) | Reactant conditions/(g·L-1) | Time/min | Degradation efficiency/% | Ref. |
|---|---|---|---|---|---|
| MIL-53(Fe)@AC | 20 | [catalyst]=0.20 [PMS]=2.00 | 120 | 92.4 | [S1] |
| Fe-N/BC | 30 | [catalyst]=0.05 [PMS]=0.50 | 60 | 89.9 | [S2] |
| Mn-MoS2@AABs | 20 | [catalyst]=0.40 [PMS]=2.50 | 90 | 82.4 | [S3] |
| Co@N-MC | 25 | [catalyst]=0.04 [PMS]=0.20 | 30 | 91.2 | [S4] |
| ZCO-CN | 20 | [catalyst]=0.13 [PMS]=0.10 | 30 | 91.0 | [S5] |
| MIL-88A/CoFe2O4 | 10 | [catalyst]=0.25 [PMS]=1.00 | 60 | 90.0 | [S6] |
| CoFe2O4/MgAl-LDH | 25 | [catalyst]=0.20* [PMS]=0.23* | 45 | 98.2 | This work |
Table S2 Comparison of the effects of different catalysts on degrading TCH
| Catalyst | Tetracycline concentration/(mg·L-1) | Reactant conditions/(g·L-1) | Time/min | Degradation efficiency/% | Ref. |
|---|---|---|---|---|---|
| MIL-53(Fe)@AC | 20 | [catalyst]=0.20 [PMS]=2.00 | 120 | 92.4 | [S1] |
| Fe-N/BC | 30 | [catalyst]=0.05 [PMS]=0.50 | 60 | 89.9 | [S2] |
| Mn-MoS2@AABs | 20 | [catalyst]=0.40 [PMS]=2.50 | 90 | 82.4 | [S3] |
| Co@N-MC | 25 | [catalyst]=0.04 [PMS]=0.20 | 30 | 91.2 | [S4] |
| ZCO-CN | 20 | [catalyst]=0.13 [PMS]=0.10 | 30 | 91.0 | [S5] |
| MIL-88A/CoFe2O4 | 10 | [catalyst]=0.25 [PMS]=1.00 | 60 | 90.0 | [S6] |
| CoFe2O4/MgAl-LDH | 25 | [catalyst]=0.20* [PMS]=0.23* | 45 | 98.2 | This work |
| [1] | HUO Y, ZHOU G, GUAN Y, et al. Inducing oxygen vacancies in ZnO/Co3O4 via g-C3N4 carrier for enhanced universality and stability in TC degradation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 683: 132974. |
| [2] | LIU Y, LIU S, CHEN M, et al. Enhanced TC degradation by persulfate activation with carbon-coated CoFe2O4: the radical and non-radical co-dominant mechanism, DFT calculations and toxicity evaluation. Journal of Hazardous Materials, 2024, 461: 132417. |
| [3] | DAI T, YUAN Z, MENG Y, et al. Performance and mechanism of photocatalytic degradation of tetracycline by Z-scheme heterojunction of CdS@LDHs. Applied Clay Science, 2021, 212: 106210. |
| [4] | FILHO F G N, FILHO E C S, OSAJIMA J A, et al. Adsorption of tetracycline using chitosan-alginate-bentonite composites. Applied Clay Science, 2023, 239: 106952. |
| [5] | ZHAO Q, YIN W, LONG C, et al. Insights into the adsorption behaviour and mechanism of tetracycline on rectorite mineral: influence of surface and structure evolution. Applied Clay Science, 2022, 229: 106698. |
| [6] | PU M, AILIJIANG N, MAMAT A, et al. Occurrence of antibiotics in the different biological treatment processes, reclaimed wastewater treatment plants and effluent-irrigated soils. Journal of Environmental Chemical Engineering, 2022, 10(3):107715. |
| [7] | ZHANG D, HE Q, HU X, et al. Enhanced adsorption for the removal of tetracycline hydrochloride (TC) using ball-milled biochar derived from crayfish shell. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 615: 126254. |
| [8] | PHOON B L, ONG C C, SAHEED M S M, et al. Conventional and emerging technologies for removal of antibiotics from wastewater. Journal of Hazardous Materials, 2020, 400: 122961. |
| [9] | XIANG W, ZHANG X, LUO J, et al. Performance of lignin impregnated biochar on tetracycline hydrochloride adsorption: governing factors and mechanisms. Environmental Research, 2022, 215: 114339. |
| [10] | MA D, YI H, LAI C, et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere, 2021, 275: 130104. |
| [11] |
NIMAI S, ZHANG H, WU Z, et al. Efficient degradation of sulfamethoxazole by acetylene black activated peroxydisulfate. Chinese Chemical Letters, 2020, 31(10):2657.
DOI |
| [12] | DHIMAN P, KUMAR A, RANA G, et al. Cobalt-zinc nanoferrite for synergistic photocatalytic and peroxymonosulfate-assisted degradation of sulfosalicylic acid. Journal of Materials Science, 2023, 58(24):9938. |
| [13] | GAO S, PAN J, ZHANG Y, et al. Mn-NSC co-doped modified biochar/permonosulfate system for degradation of ciprofloxacin in wastewater. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 680: 132640. |
| [14] | HAN Y, ZHU Z, HU C, et al. 3D flower-like Cu-BiOCl/Bi2S3 heterostructure with synergistic Cu ion doping: a study on efficient tetracycline degradation under visible light. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 683: 133014. |
| [15] | KOHANTORABI M, MOUSSAVI G, GIANNAKIS S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chemical Engineering Journal, 2021, 411: 127957. |
| [16] | YOU Y, XU G, YANG X, et al. Cu-Fe-Ni layered hydroxides/ magnetic biochar composite as peroxymonosulfate activator for removal of enrofloxacin. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 683: 133082. |
| [17] | KHAGHANI R, KAKAVANDI B, GHADIRINEJAD K, et al. Preparation, characterization and catalytic potential of γ-Fe2O3@AC mesoporous heterojunction for activation of peroxymonosulfate into degradation of cyfluthrin insecticide. Microporous and Mesoporous Materials, 2019, 284: 111. |
| [18] | REZAEI S S, DEHGHANIFARD E, NOORISEPEHR M, et al. Efficient clean-up of waters contaminated with diazinon pesticide using photo-decomposition of peroxymonosulfate by ZnO decorated on a magnetic core/shell structure. Journal of Environmental Management, 2019, 250: 109472. |
| [19] | WANG J, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chemical Engineering Journal, 2018, 334: 1502. |
| [20] | TAN Y, LI C, SUN Z, et al. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate. Chemical Engineering Journal, 2020, 388: 124386. |
| [21] | LIU L, ZHAN R, ZHANG M, et al. Insights into the performance, mechanism, and ecotoxicity of levofloxacin degradation in CoFe2O4 catalytic peroxymonosulfate process. Journal of Environmental Chemical Engineering, 2022, 10(3):107435. |
| [22] | GAN L, ZHONG Q, GENG A, et al. Cellulose derived carbon nanofiber: a promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Science of the Total Environment, 2019, 694: 133705. |
| [23] | ZHU J, WANG S, YANG Z, et al. Robust polystyrene resin- supported nano-CoFe2O4 mediated peroxymonosulfate activation for efficient oxidation of 1-hydroxyethane 1,1-diphosphonic acid. Journal of Hazardous Materials, 2023, 443: 130281. |
| [24] | FAN Y, LIU Y, HU X, et al. Preparation of metal organic framework derived materials CoFe2O4@NC and its application for degradation of norfloxacin from aqueous solutions by activated peroxymonosulfate. Chemosphere, 2021, 275: 130059. |
| [25] | SUN Y, ZHOU J, LIU D, et al. Highly efficient removal of tetracycline hydrochloride under neutral conditions by visible photo-Fenton process using novel MnFe2O4/diatomite composite. Journal of Water Process Engineering, 2021, 43: 102307. |
| [26] | WANG Y, KANG X, LI Y, et al. Cobalt-loaded carbon nanofibers as magnetic catalyst for tetracycline degradation through peroxydisulfate activation: non-radical dominated mechanism. Journal of Water Process Engineering, 2024, 57: 104600. |
| [27] | FEI W, SONG Y, LI N, et al. Fabrication of visible-light-active ZnO/ZnFe-LDH heterojunction on Ni foam for pollutants removal with enhanced photoelectrocatalytic performance. Solar Energy, 2019, 188: 593. |
| [28] | CHAGAS C A, DE SOUZA E F, DE CARVALHO M C N A, et al. Cobalt ferrite nanoparticles for the preferential oxidation of CO. Applied Catalysis A: General, 2016, 519: 139. |
| [29] | ZHANG M, TAO H, ZHAI C, et al. Twin-brush ZnO mesocrystal for the piezo-activation of peroxymonosulfate to remove ibuprofen in water: performance and mechanism. Applied Catalysis B: Environmental, 2023, 326: 122399. |
| [30] | WANG L, LI J, DU Z, et al. MnFe2O4/zeolite composite catalyst for activating peroxymonosulfate to efficiently degrade antibiotic. Materials Letters, 2023, 344: 134460. |
| [31] | AL-GHOUTI M A, DA'ANA D A. Guidelines for the use and interpretation of adsorption isotherm models: a review. Journal of Hazardous Materials, 2020, 393: 122383. |
| [32] | HUANG N, WANG T, WU Y, et al. Preparation of magnetically recyclable hierarchical porous sludge-pine needle derived biochar loaded CoFe2O4 nanoparticles for rapid degradation of tetracycline by activated PMS. Materials Today Communications, 2023, 35: 106313. |
| [33] | WANG X, CHENG B, ZHANG L, et al. Synthesis of MgNiCo LDH hollow structure derived from ZIF-67 as superb adsorbent for Congo red. Journal of Colloid and Interface Science, 2022, 612: 598. |
| [34] | QIN Q, WU X, CHEN L, et al. Simultaneous removal of tetracycline and Cu(Ⅱ) by adsorption and coadsorption using oxidized activated carbon. RSC Advances, 2018, 8(4):1744. |
| [35] | FARGHALI M A, SELIM A M, KHATER H F, et al. Optimized adsorption and effective disposal of Congo red dye from wastewater: hydrothermal fabrication of MgAl-LDH nanohydrotalcite- like materials. Arabian Journal of Chemistry, 2022, 15(11):104171. |
| [36] | LI Y, MA S, XU S, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chemical Engineering Journal, 2020, 387: 124094. |
| [1] | WANG Lei, LI Jianjun, NING Jun, HU Tianyu, WANG Hongyang, ZHANG Zhanqun, WU Linxin. Enhanced Degradation of Methyl Orange with CoFe2O4@Zeolite Catalyst as Peroxymonosulfate Activator: Performance and Mechanism [J]. Journal of Inorganic Materials, 2023, 38(4): 469-476. |
| [2] | TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. |
| [3] | LAN Qing, SUN Shengrui, WU Ping, YANG Qingfeng, LIU Yangqiao. Co-doped CuO/Visible Light Synergistic Activation of PMS for Degradation of Rhodamine B and Its Mechanism [J]. Journal of Inorganic Materials, 2021, 36(11): 1171-1177. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||