
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (12): 1331-1338.DOI: 10.15541/jim20240206
Special Issue: 【能源环境】储能电池(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
KONG Jianfeng1( ), HUANG Jiecheng1, LIU Zhaolin2, LIN Cunsheng2(
), HUANG Jiecheng1, LIU Zhaolin2, LIN Cunsheng2( ), WANG Zhiyu1,2(
), WANG Zhiyu1,2( )
)
Received:2024-04-22
Revised:2024-05-09
Published:2024-06-24
Online:2024-06-24
Contact:
WANG Zhiyu, professor. E-mail: zywang@dlut.edu.cn;About author:KONG Jianfeng (1999-), male, Master candidate. E-mail: fengfeng1014@mail.dlut.edu.cn
Supported by:CLC Number:
KONG Jianfeng, HUANG Jiecheng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on DPEPA-derived Gel Polymer Electrolyte[J]. Journal of Inorganic Materials, 2024, 39(12): 1331-1338.
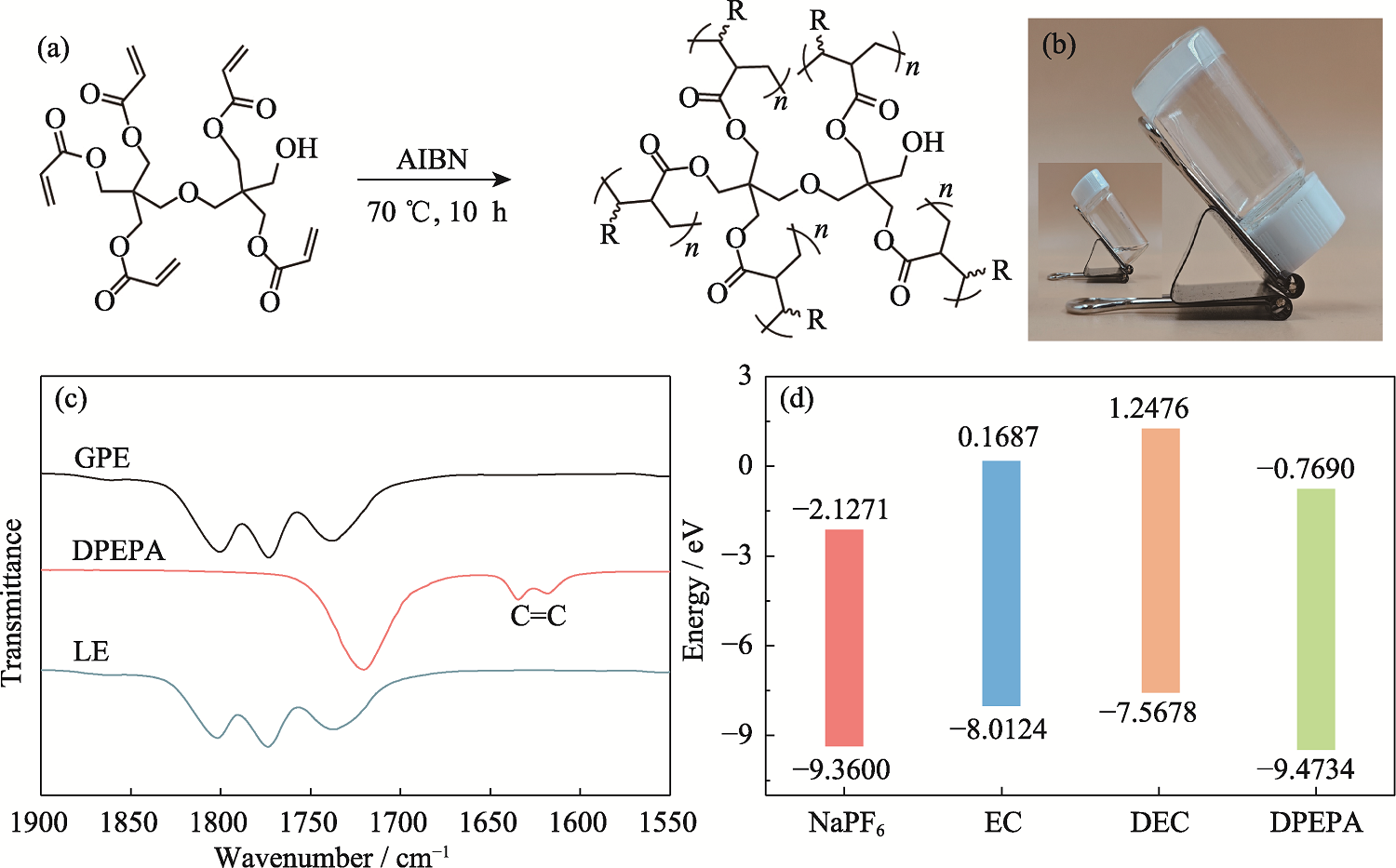
Fig. 1 Synthesis and structure of GPE (a) Thermal-driven radical in-situ polymerization of DPEPA; (b) Optical image of DPEPA-based GPE; (c) FT-IR spectra of GPE, DPEPA and liquid electrolyte (LE); (d) HOMO and LUMO energy levels of NaPF6, EC, DEC and DPEPA
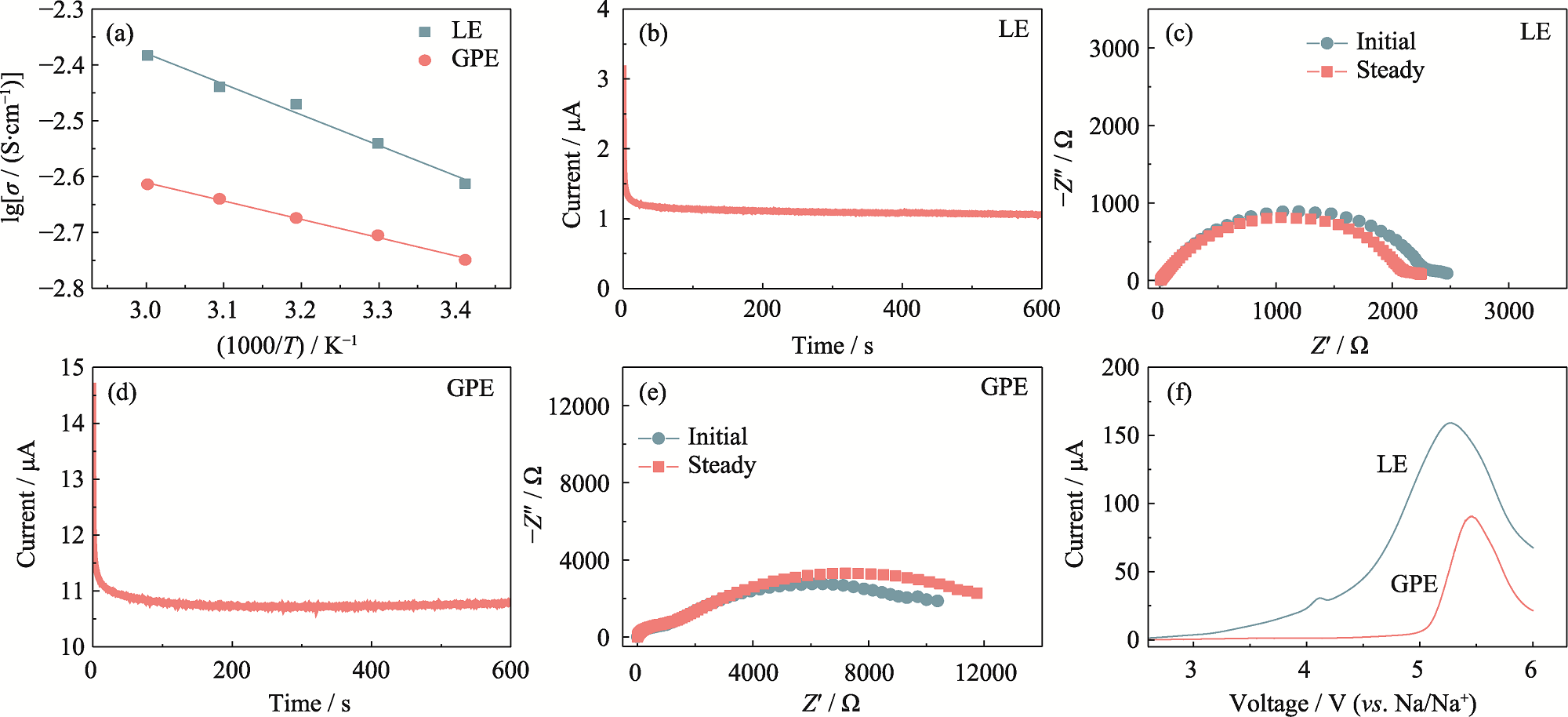
Fig. 2 Electrochemical performance of GPE and LE (a) Ionic conductivities of LE and GPE at various temperatures; (b) Chronoamperometry profile and (c) Nyquist plot of LE; (d) Chronoamperometry profile and (e) Nyquist plot of GPE; (f) LSV curves of LE and GPE at a scan rate of 1 mV·s-1
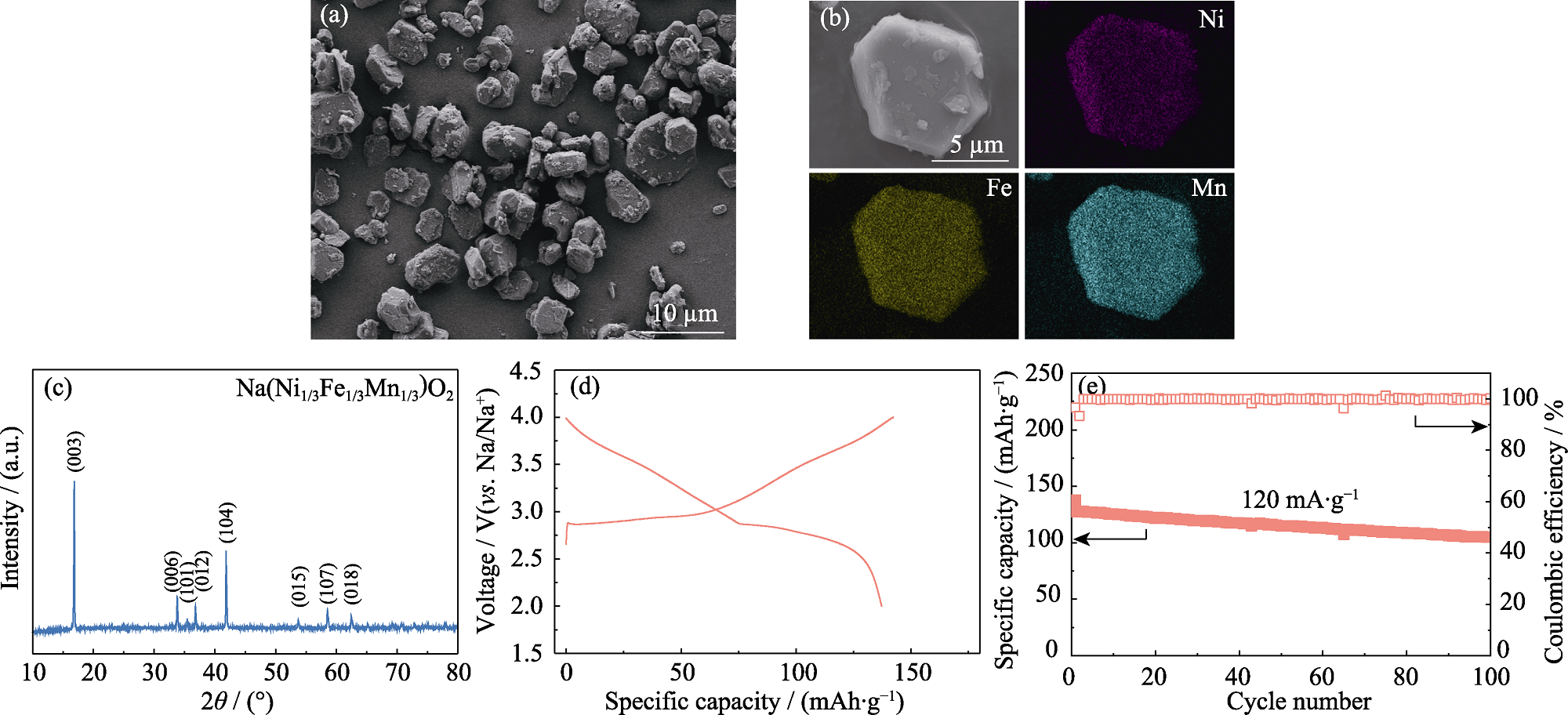
Fig. 3 Morphology and structure of NFM cathode, as well as its electrochemical performance in half cell (a) SEM image, (b) elemental mappings and (c) XRD pattern of NFM; (d) Charge-discharge curves of NFM cathode at a current density of 12 mA·g-1; (e) Cycling performance of NFM cathode at 120 mA·g-1
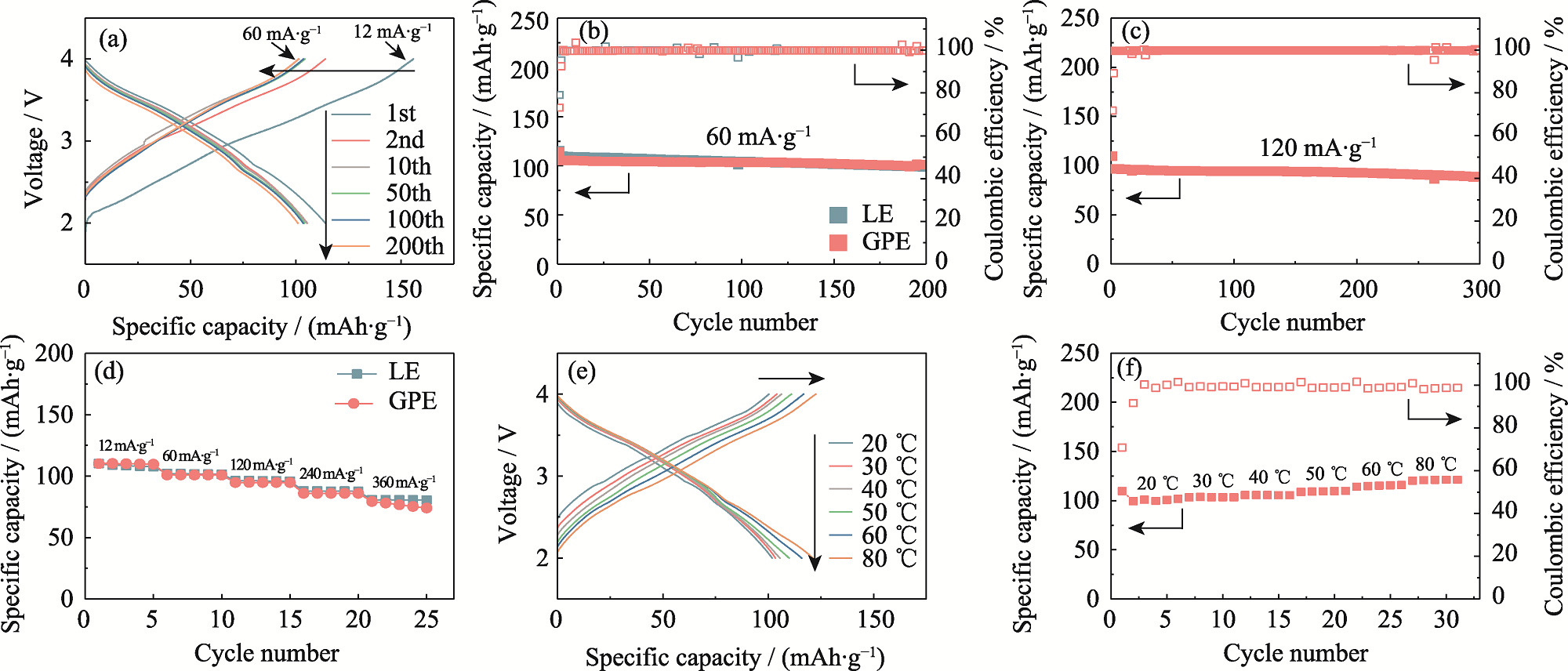
Fig. 4 Electrochemical performance of quasi-solid-state Na-ion full cell using NFM cathode and HC anode in GPE or LE (a) Charge-discharge curves of the full cell using GPE at a current density of 60 mA·g-1 with the first cycle conducted at a current density of 12 mA·g-1; (b) Cycling performance of the full cell using LE or GPE at 60 mA·g-1; (c) Cycling performance of the full cell using GPE at 120 mA·g-1; (d) Rate capability of the full cell using LE or GPE at various current densities of 12-360 mA·g-1; (e) Charge-discharge curves and (f) capacity retention of the full cell using GPE in a temperature range of 20-80 ℃ at 60 mA·g-1. Colorful figures are available on website
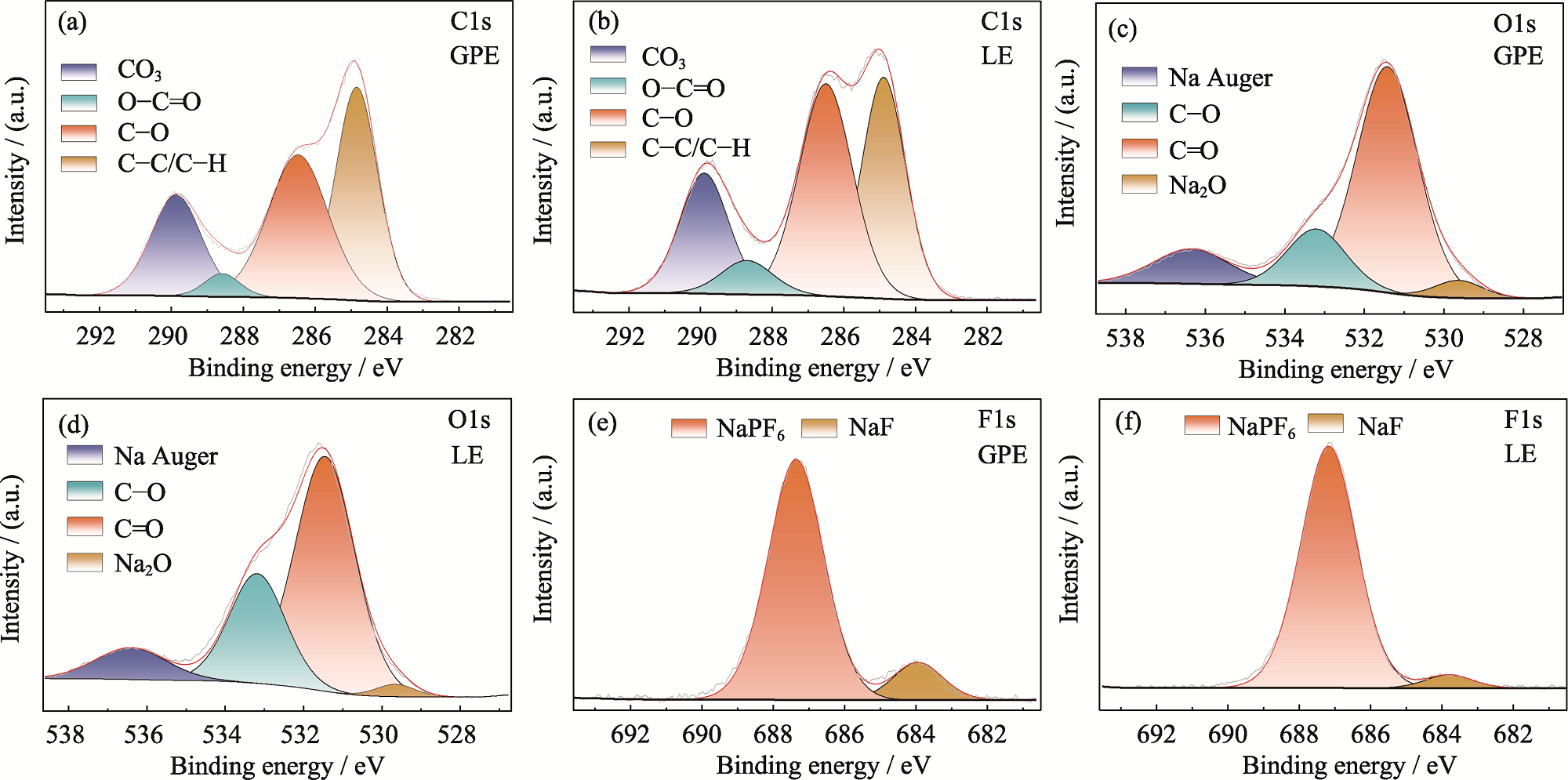
Fig. 5 Chemical composition of SEI films on HC anode after cycling in GPE or LE High-resolution (a, b) C1s, (c, d) O1s and (e, f) F1s XPS spectra of SEI film formed in (a, c, e) GPE or (b, d, f) LE Colorful figures are available on website
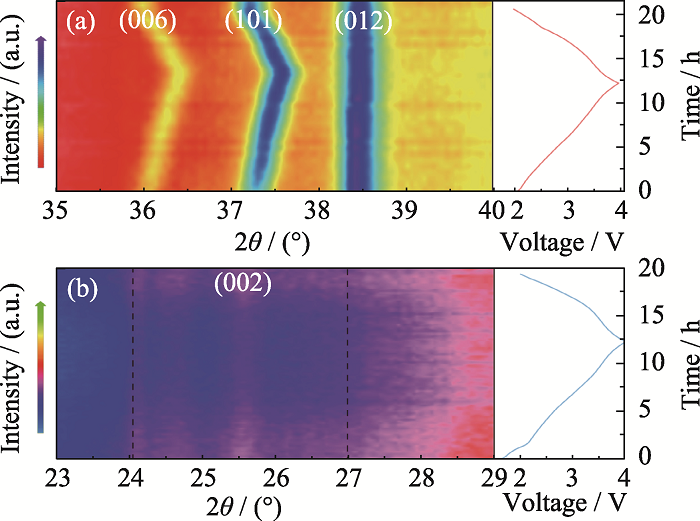
Fig. 6 In-situ analysis of the mechanism for Na storage of NFM cathode and HC anode in Na-ion full cell In-situ XRD patterns of (a) NFM cathode and (b) HC anode within a voltage range of 2.0-4.0 V
| [1] |
LIU Y Z, MENG X Y, WANG Z Y, et al. Development of quasi-solid-state anode-free high-energy lithium sulfide-based batteries. Nature Communications, 2022, 13(1):4415.
DOI PMID |
| [2] | LIAO Y Q, YUAN L X, HAN Y, et al. Pentafluoro (phenoxy) cyclotriphosphazene stabilizes electrode/electrolyte interfaces for sodium-ion pouch cells of 145 Wh kg-1. Advanced Materials, 2024, 36(16):e2312287. |
| [3] | ZHAO A, FANG Y J, AI X P, et al. Mixed polyanion cathode materials: toward stable and high-energy sodium-ion batteries. Journal of Energy Chemistry, 2021, 60: 635. |
| [4] | WANG X, HUANG H J, ZHOU F, et al. High-voltage aqueous planar symmetric sodium ion micro-batteries with superior performance at low-temperature of -40 ℃. Nano Energy, 2021, 82: 105688. |
| [5] | NIMKAR A, SHPIGEL N, MALCHIK F, et al. Unraveling the role of fluorinated alkyl carbonate additives in improving cathode performance in sodium-ion batteries. ACS Applied Materials & Interfaces, 2021, 13(39):46478. |
| [6] | LI W K, ZHAO N, BI Z J, et al. Na3Zr2Si2PO12 ceramic electrolytes for Na-ion battery: preparation using spray-drying method and its property. Journal of Inorganic Materials, 2022, 37(2):189. |
| [7] | LIANG J N, LUO J, SUN Q, et al. Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries. Energy Storage Materials, 2019, 21: 308. |
| [8] |
LI D, LEI C, LAI H, et al. Recent advancements in interface between cathode and garnet solid electrolyte for all solid state Li-ion batteries. Journal of Inorganic Materials, 2019, 34(7):694.
DOI |
| [9] | BI Z J, SHI R D, LIU X N, et al. In situ conversion reaction triggered alloy@antiperovskite hybrid layers for lithiophilic and robust lithium/garnet interfaces. Advanced Functional Materials, 2023, 33(43):2307701. |
| [10] | WEN P C, LU P F, SHI X Y, et al. Photopolymerized gel electrolyte with unprecedented room-temperature ionic conductivity for high- energy-density solid-state sodium metal batteries. Advanced Energy Materials, 2021, 11(6):2002930. |
| [11] | BI Z J, HUANG W L, MU S, et al. Dual-interface reinforced flexible solid garnet batteries enabled by in-situ solidified gel polymer electrolytes. Nano Energy, 2021, 90: 106498. |
| [12] | BI Z J, SUN Q F, JIA M Y, et al. Molten salt driven conversion reaction enabling lithiophilic and air-stable garnet surface for solid-state lithium batteries. Advanced Functional Materials, 2022, 32(52):2208751. |
| [13] | ZHANG T W, ZHANG J, YANG S, et al. Facile in situ chemical cross-linking gel polymer electrolyte, which confines the shuttle effect with high ionic conductivity and Li-ion transference number for quasi-solid-state lithium-sulfur battery. ACS Applied Materials & Interfaces, 2021, 13(37):44497. |
| [14] | SWORAKOWSKI J, LIPINSKI J, JANUS K. On the reliability of determination of energies of HOMO and LUMO levels in organic semiconductors from electrochemical measurements. A simple picture based on the electrostatic model. Organic Electronics, 2016, 33: 300. |
| [15] |
ZHOU L, CAO Z, ZHANG J, et al. Engineering sodium-ion solvation structure to stabilize sodium anodes: universal strategy for fast-charging and safer sodium-ion batteries. Nano Letters, 2020, 20(5):3247.
DOI PMID |
| [16] | ZHANG L B, DESHMUKH J, HIJAZI H, et al. Impact of calcium on air stability of Na[Ni1/3Fe1/3Mn1/3]O2 positive electrode material for sodium-ion batteries. Journal of the Electrochemical Society, 2023, 170(7):070514. |
| [17] | FONDARD J, IRISARRI E, COURRÈGES C, et al. SEI composition on hard carbon in Na-ion batteries after long cycling: influence of salts (NaPF6, NaTFSI) and additives (FEC, DMCF). Journal of the Electrochemical Society, 2020, 167(7):070526. |
| [18] |
ZHAO S W, HUANG F Q. Weakly solvating few-layer-carbon interface toward high initial coulombic efficiency and cyclability hard carbon anodes. ACS Nano, 2024, 18(2):1733.
DOI PMID |
| [19] | LIU M Q, WU F, GONG Y T, et al. Interfacial-catalysis-enabled layered and inorganic-rich SEI on hard carbon anodes in ester electrolytes for sodium-ion batteries. Advanced Materials, 2023, 35(29):2300002. |
| [20] | ZHOU X Z, ZHANG Q, ZHU Z, et al. Anion-reinforced solvation for a gradient inorganic-rich interphase enables high-rate and stable sodium batteries. Angewandte Chemie International Edition, 2022, 61(30):e202205045. |
| [21] | LU Z Y, GENG C N, YANG H J, et al. Step-by-step desolvation enables high-rate and ultra-stable sodium storage in hard carbon anodes. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(40):e2210203119. |
| [22] | LI Y M, HU Y S, TITIRICI M M, et al. Hard carbon microtubes made from renewable cotton as high-performance anode material for sodium-ion batteries. Advanced Energy Materials, 2016, 6(18):1600659. |
| [23] | LI Y, HU Y S, QI X, et al. Advanced sodium-ion batteries using superior low cost pyrolyzed anthracite anode: towards practical applications. Energy Storage Materials, 2016, 5: 191. |
| [24] |
TANG Z, ZHANG R, WANG H Y, et al. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery. Nature Communications, 2023, 14(1):6024.
DOI PMID |
| [1] | WANG Kunpeng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on Water-minimal Prussian Blue Cathode [J]. Journal of Inorganic Materials, 2024, 39(9): 1005-1012. |
| [2] | KONG Guoqiang, LENG Mingzhe, ZHOU Zhanrong, XIA Chi, SHEN Xiaofang. Sb Doped O3 Type Na0.9Ni0.5Mn0.3Ti0.2O2 Cathode Material for Na-ion Battery [J]. Journal of Inorganic Materials, 2023, 38(6): 656-662. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||