
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (4): 404-412.DOI: 10.15541/jim20210261
• RESEARCH ARTICLE • Previous Articles Next Articles
MA Hui1( ), TAO Jianghui1, WANG Yanni1, HAN Yu1, WANG Yabin1,2(
), TAO Jianghui1, WANG Yanni1, HAN Yu1, WANG Yabin1,2( ), DING Xiuping3(
), DING Xiuping3( )
)
Received:2021-04-20
Revised:2021-07-11
Published:2022-04-20
Online:2021-07-12
Contact:
WANG Yabin, associate professor. E-mail: ybw_bingerbingo@126.com;About author:MA Hui (1996-), female, Master candidate. E-mail: mh08201@163.com
Supported by:CLC Number:
MA Hui, TAO Jianghui, WANG Yanni, HAN Yu, WANG Yabin, DING Xiuping. Gold Nanoparticles Supported on Silica & Titania Hybrid Mesoporous Spheres and Their Catalytic Performance Regulation[J]. Journal of Inorganic Materials, 2022, 37(4): 404-412.
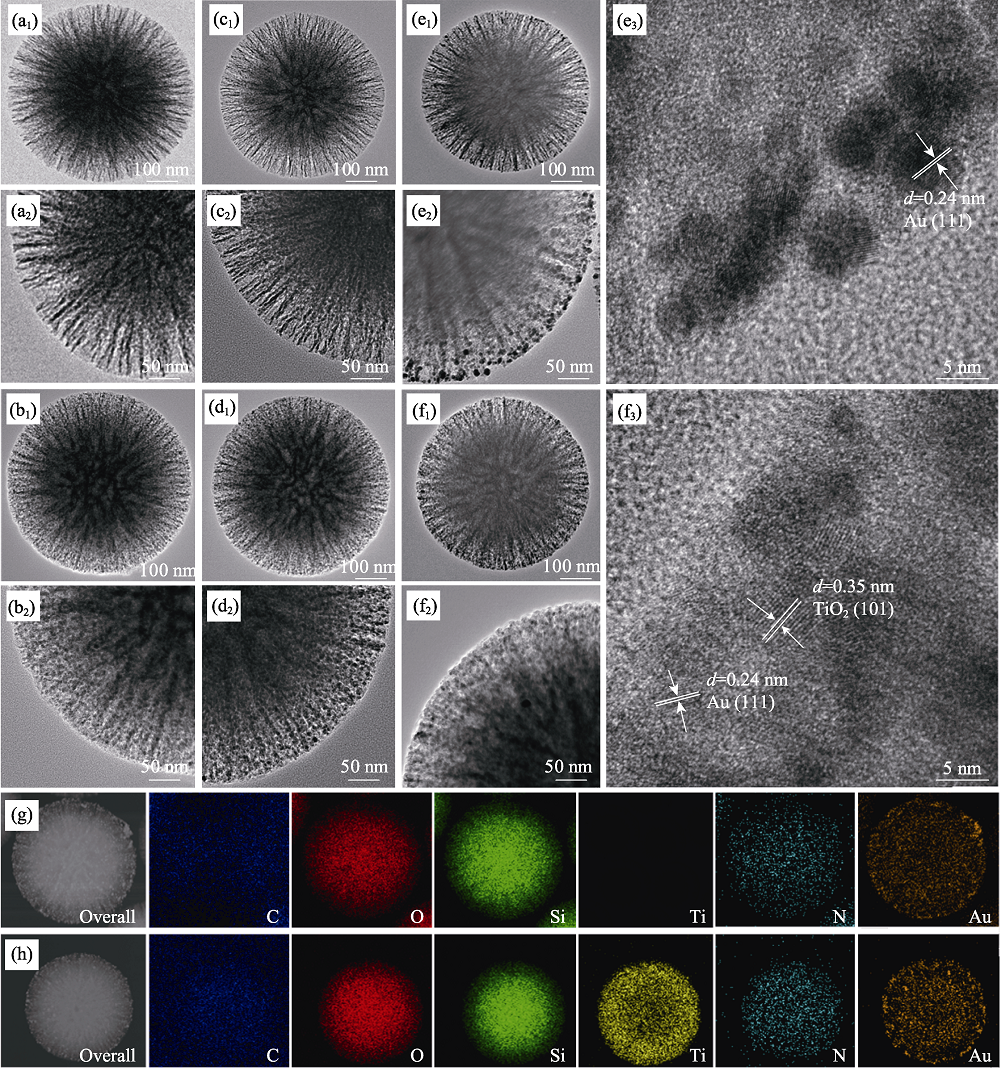
Fig.5 TEM images of DMSNs (a), DMSTNs (b), DMSNs-NH2 (c), DMSTNs-NH2 (d), DMSNs-NH2-Au (e), and DMSTNs-NH2-Au (f), and TEM-mapping images of DMSNs-NH2-Au (g) and DMSTNs-NH2-Au (h)
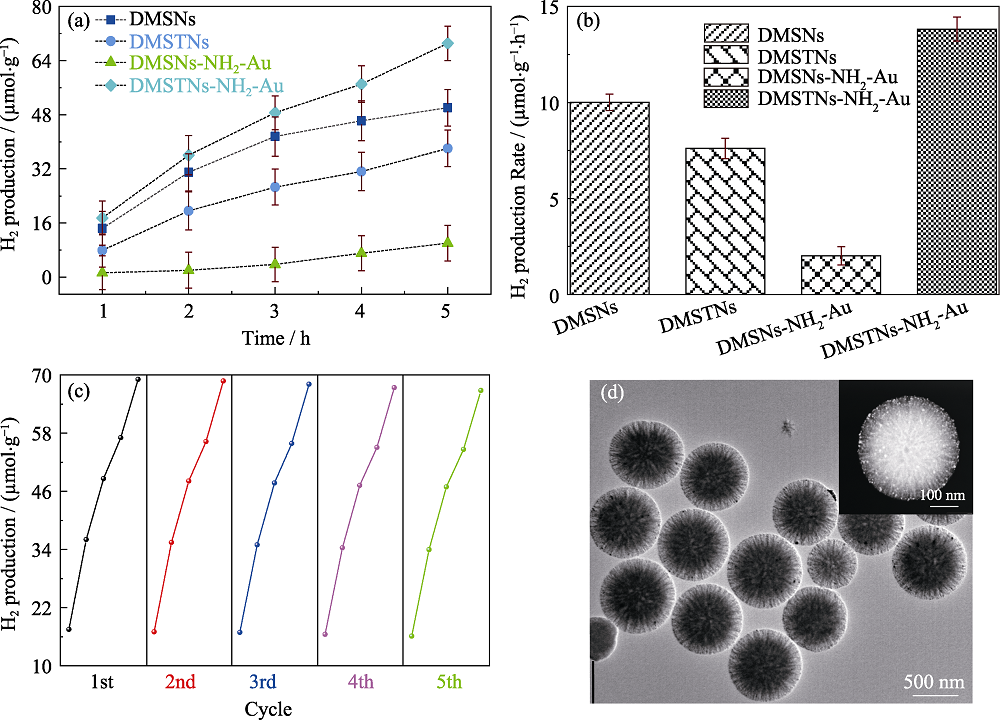
Fig. 7 H2 production amount as a function of irradiation time (a) and the corresponding production rates (b) of DMSNs, DMSTNs, DMSNs-NH2-Au, and DMSTNs-NH2-Au, cycling tests of DMSTNs-NH2-Au for H2 production (c), and TEM image of DMSTNs-NH2-Au sample experienced five cycles, with inset showing the high-angle annular dark-field imaging (d)
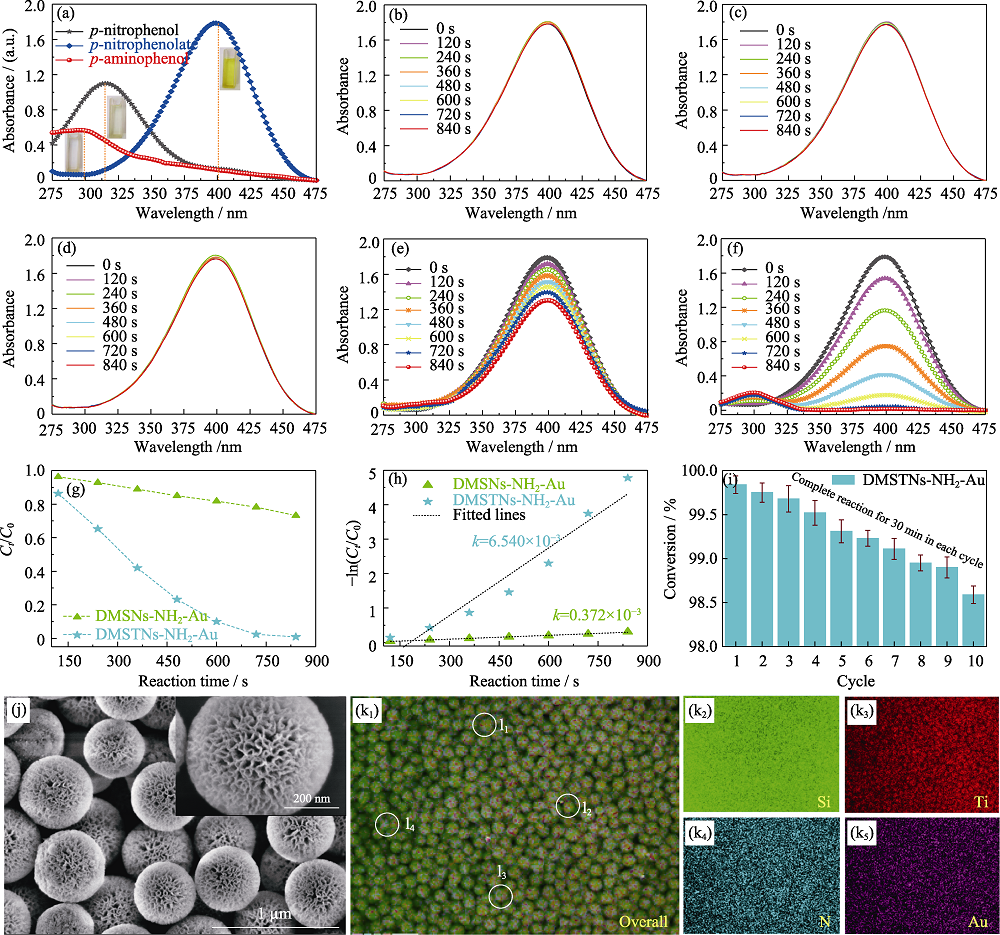
Fig. 8 Characteristic ultraviolet absorption peaks of p-nitrophenol, p-nitrophenolate, and p-aminophenol (a), Ultraviolet absorption spectra of different samples, including p-nitrophenol+NaBH4 as the blank sample (b), with the addition of DMSNs (c), DMSTNs (d), DMSNs-NH2-Au (e), and DMSTNs-NH2-Au (f), the conversion (g) and pseudo first-order linear equation (h) of DMSNs-NH2-Au, and DMSTNs-NH2-Au, and cycling tests of DMSTNs-NH2-Au for p-nitrophenol reduction (i), SEM images (j) and energy dispersive spectroscopy (EDS) mappings (k) of DMSTNs-NH2-Au sample experienced ten cycles. EDS measurement of random four DMSTNs-NH2-Au individuals (l)
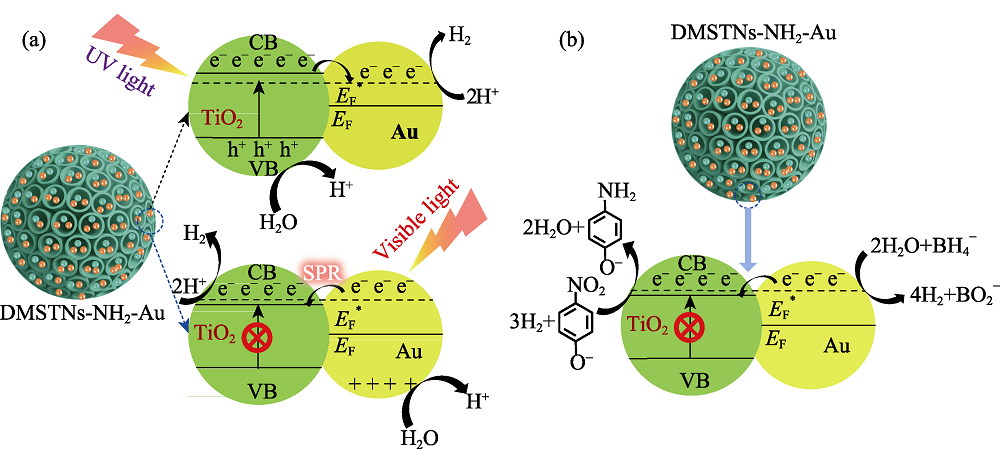
Fig. 9 Schematic illustration of possible photocatalytic mechanisms for DMSTNs-NH2-Au to split water under simulated sunlight (a) and ordinary catalytic reduction of p-nitrophenol without light irritation (b)
| [1] |
MEEMKEN F, BAIKER A. Recent progress in heterogeneous asymmetric hydrogenation of C=O and C=C bonds on supported noble metal catalysts. Chemical Reviews, 2017, 117(17): 11522-11569.
DOI URL |
| [2] |
DHIMAN M, CHALKE B, POLSHETTIWAR V. Organosilane oxidation with a half million turnover number using fibrous nanosilica supported ultrasmall nanoparticles and pseudo-single atoms of gold. Journal of Materials Chemistry A, 2017, 5(5): 1935-1940.
DOI URL |
| [3] |
SANTOS V P, CARABINEIRO S A C, TAVARES P B, et al. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Applied Catalysis B: Environmental, 2010, 99(1): 198-205.
DOI URL |
| [4] |
LIU M, HOF F, MORO M, et al. Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting. Nanoscale, 2020, 12(39): 20165-20170.
DOI URL |
| [5] |
ZHANG Y P, ZHOU Y, ZHAO Y, et al. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catalysis Today, 2016, 263: 61-68.
DOI URL |
| [6] | KASHYAP T, BISWASI S, PAL A R, et al. Unraveling the catalytic and plasmonic roles of g-C3N4 supported Ag and Au nanoparticles under selective photoexcitation. ACS Sustainable Chemistry & Engineering, 2019, 7(23): 19295-19302. |
| [7] |
MAO S J, WANG C P, WANG Y. The chemical nature of N doping on N doped carbon supported noble metal catalysts. Journal of Catalysis, 2019, 375: 456-465.
DOI URL |
| [8] |
XU J, ZHANG J Y, PENG H G, et al. Ag supported on meso- structured SiO2 with different morphologies for CO oxidation: on the inherent factors influencing the activity of Ag catalysts. Microporous and Mesoporous Materials, 2017, 242: 90-98.
DOI URL |
| [9] |
LE X D, DONG Z P, LIU Y S, et al. Palladium nanoparticles immobilized on core-shell magnetic fibers as a highly efficient and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol and Suzuki coupling reactions. Journal of Materials Chemistry A, 2014, 2(46): 19696-19706.
DOI URL |
| [10] |
SINGH B, POLSHETTIWAR V. Design of CO2 sorbents using functionalized fibrous nanosilica (KCC-1): insights into the effect of the silica morphology (KCC-1 vs. MCM-41). Journal of Materials Chemistry A, 2016, 4(18): 7005-7019.
DOI URL |
| [11] |
SINGH R, BAPAT R, QIN L, et al. Atomic layer deposited (ALD) TiO2 on fibrous nano-silica (KCC-1) for photocatalysis: nanoparticle formation and size quantization effect. ACS Catalysis, 2016, 6(5): 2770-2784.
DOI URL |
| [12] |
MAITY A, BELGAMWAR R, POLSHETTIWAR V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nature Protocols, 2019, 14: 2177-2204.
DOI URL |
| [13] | POLSHETTIWAR V, CHA D, ZHANG X, et al. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology. Angewandte Chemie International Edition, 2010, 49(50): 9652-9656. |
| [14] |
WANG Y, TANG J, YANG Y N, et al. Functional nanoparticles with a reducible tetrasulfide motif to upregulate mRNA translation and enhance transfection in hard-to-transfect cells. Angewandte Chemie International Edition, 2020, 59(7): 2695-2699.
DOI URL |
| [15] | DONG Z P, LE X D, LI X L, et al. Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Applied Catalysis B Environmental, 2014, 158: 129-135. |
| [16] |
DONG Z P, LE X D, DONG C X, et al. Ni@Pd core-shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Applied Catalysis B Environmental, 2015, 162: 372-380.
DOI URL |
| [17] |
LE X D, DONG Z P, ZHANG W, et al. Fibrous nano-silica containing immobilized Ni@Au core-shell nanoparticles: a highly active and reusable catalyst for the reduction of 4-nitrophenol and 2-nitroaniline. Journal of Molecular Catalysis A: Chemical, 2014, 395: 58-65.
DOI URL |
| [18] |
WANG Y B, HE J, SHI Y M, et al. Structure- dependent adsorptive or photocatalytic performances of solid and hollow dendritic mesoporous silica&titania nanospheres. Microporous and Mesoporous Materials, 2020, 305 110326.
DOI URL |
| [19] |
WANG Y B, HE J, LI X L, et al. Dendritic mesoporous silica&titania nanospheres (DMSTNs) coupled with amorphous carbon nitride (ACN) for improved visible-light-driven hydrogen production. Applied Surface Science, 2021, 538 148147.
DOI URL |
| [20] |
WANG Y B, HU K K, HE J, et al. Improving the size uniformity of dendritic fibrous nano-silica by a facile one-pot rotating hydrothermal approach. RSC Advances, 2019, 9(43): 24783-24790.
DOI URL |
| [21] |
FIHRI A, CHA D, BOUHRARA M, et al. Fibrous nano-silica (KCC-1)-supported palladium catalyst: Suzuki coupling reactions under sustainable conditions. ChemSusChem, 2012, 5(1): 85-89.
DOI URL |
| [22] |
SADEGHZADEH S M. A heteropolyacid-based ionic liquid immobilized onto fibrous nano-silica as an efficient catalyst for the synthesis of cyclic carbonate from carbon dioxide and epoxides. Green Chemistry, 2015, 17(5): 3059-3066.
DOI URL |
| [23] |
REN J, LI Z, LIU S S, et al. Silica-titania mixed oxides: Si-O-Ti connectivity, coordination of titanium, and surface acidic properties. Catalysis Letters, 2008, 124(34): 185-194.
DOI URL |
| [24] |
VAIANO V, MATARANGOLO M, MURCIA J J, et al. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Applied Catalysis B: Environmental, 2018, 225: 197-206.
DOI URL |
| [25] |
JING L Q, QU Y C, WANG B Q, et al. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Solar Energy Materials and Solar Cells, 2006, 90(12): 1773-1787.
DOI URL |
| [26] |
HAMID M Y S, FIRMANSYAH M L, TRIWAHYONO S, et al. Oxygen vacancy-rich mesoporous silica KCC-1 for CO2 methanation. Applied Catalysis A General, 2017, 532: 86-94.
DOI URL |
| [27] |
JAKOB M, LEVANON H, KAMAT P V. Charge distribution between UV-irradiated TiO2 and gold nanoparticles: determination of shift in the fermi level. Nano Letters, 2003, 3(3): 353-358.
DOI URL |
| [28] |
SUBRAMANIAN V, WOLF E E, KAMAT P V. Catalysis with TiO2/gold nanocomposites. effect of metal particle size on the fermi level equilibration. Journal of the American Chemical Society, 2004, 126(15): 4943-4950.
DOI URL |
| [29] |
CYBULA A, PRIEBE J B, POHL M M, et al. The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Applied Catalysis B: Environmental, 2014, 152-153: 202-211.
DOI URL |
| [30] | LIU B, JIANG Y, WANG Y, et al. Influence of dimensionality and crystallization on visible-light hydrogen production of Au@TiO2 core-shell photocatalysts based on localized surface plasmon resonance. Catalysis Science&Technology, 2018, 8(4): 1094-1103. |
| [31] |
MISRA M, CHOWDHURY S R, LEE T I. Sunlight driven decomposition of toxic organic compound, coumarin, p-nitrophenol, and photo reduction of Cr(VI) ions, using a bridge structure of Au@CNT@TiO2 nanocomposite. Applied Catalysis B: Environmental, 2020, 272: 118991.
DOI URL |
| [32] |
ISMAIL A A, HAKKI A, BAHNEMANN D W. Mesostructure Au/TiO2 nanocomposites for highly efficient catalytic reduction of p-nitrophenol. Journal of Molecular Catalysis A: Chemical, 2012, 358: 145-151.
DOI URL |
| [33] |
HUANG M Q, ZHANG Y W, ZHOU Y M, et al. Synthesis and characterization of hollow ZrO2-TiO2/Au spheres as a highly thermal stability nanocatalyst. Journal of Colloid and Interface Science, 2017, 497: 23-32.
DOI URL |
| [1] | LI Sheng-Song, ZHENG Yong-Chao, MENG Shu-Lin, WU Li-Zhu, ZHONG Jin- Yi, ZHAO Chong-Lin. Core/Shell Quantum Dots and Au Nanoparticles Assembly for Effective Detection of Nerve Agent Mimic [J]. Journal of Inorganic Materials, 2019, 34(8): 893-898. |
| [2] | LIU Shu-Xia,HE Jun-Hui. Facile Fabrication of Porous Titania Microtube Arrays by Replication of Human Hair [J]. Journal of Inorganic Materials, 2006, 21(6): 1313-1318. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||