
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (10): 1031-1038.DOI: 10.15541/jim20200690
• RESEARCH ARTICLE • Previous Articles Next Articles
TANG Jiawei1( ), WANG Yongbang1,2, MA Cheng1, YANG Haixiao1, WANG Jitong1, QIAO Wenming1(
), WANG Yongbang1,2, MA Cheng1, YANG Haixiao1, WANG Jitong1, QIAO Wenming1( ), LING Licheng1
), LING Licheng1
Received:2020-12-01
Revised:2021-02-22
Published:2021-10-20
Online:2021-03-12
Contact:
QIAO Wenming, professor. E-mail: qiaowm@ecust.edu.cn
About author:TANG Jiawei (1996-), male, Master candidate. E-mail: 15216726632@163.com
Supported by:CLC Number:
TANG Jiawei, WANG Yongbang, MA Cheng, YANG Haixiao, WANG Jitong, QIAO Wenming, LING Licheng. Methylnaphthalene Pitch-based Ordered Mesoporous Carbon: Synthesis and Electrochemical Properties[J]. Journal of Inorganic Materials, 2021, 36(10): 1031-1038.
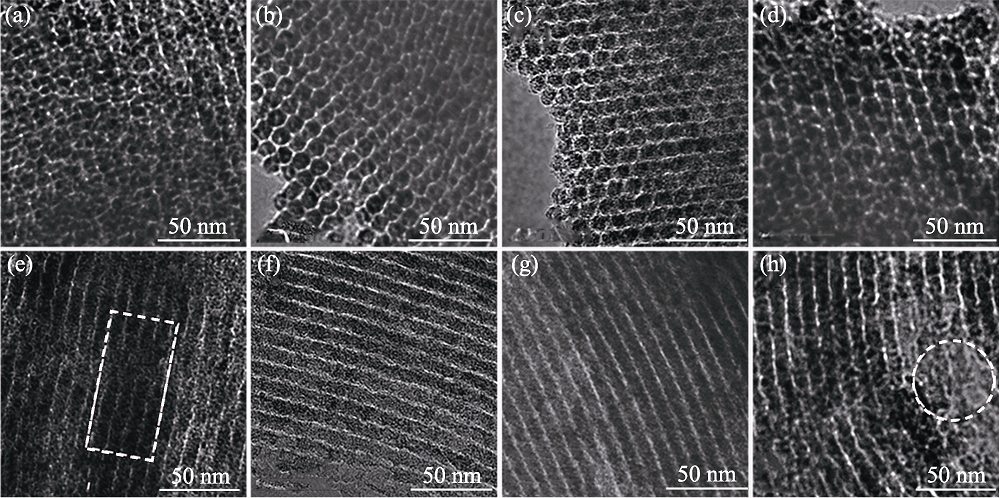
Fig. 2 TEM images of mesoporous carbon along (a-d) [100] and (e-h) [001] orientations obtained at template/pitch mass ratios of (a, e) 0.6, (b, f) 0.8, (c, g) 1.0 and (d, h) 1.2
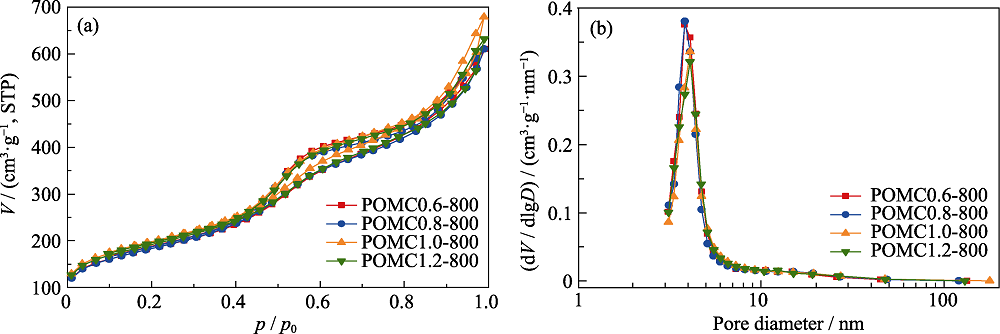
Fig. 3 (a) N2 adsorption-desorption isotherms and (b) BJH pore size distributions of mesoporous carbon obtained from various addition amounts of SBA-15
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Davea/nm | d100b/nm | a0c/nm | Wall thicknessd/nm |
|---|---|---|---|---|---|---|
| SBA-15e | 488 | 1.24 | 8.31 | 10.23 | 11.81 | 3.50 |
| POMC0.6-800 | 654 | 0.94 | 3.84 | 9.71 | 11.21 | 7.37 |
| POMC0.8-800 | 653 | 0.94 | 3.85 | 9.71 | 11.21 | 7.36 |
| POMC1.0-800 | 703 | 1.05 | 4.11 | 9.48 | 10.95 | 6.84 |
| POMC1.2-800 | 684 | 0.98 | 4.10 | 8.73 | 10.08 | 5.98 |
| POMC1.0-800-F | 681 | 0.93 | 3.85 | 8.72 | 10.07 | 6.22 |
| POMC1.0-700 | 749 | 1.16 | 4.12 | 9.46 | 10.92 | 6.80 |
| POMC1.0-900 | 675 | 1.00 | 3.84 | 9.28 | 10.72 | 6.88 |
| POMC1.0-1000 | 676 | 0.95 | 3.85 | 8.71 | 10.06 | 6.21 |
Table 1 Porous structural parameters of mesoporous carbon obtained under different process conditions
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | Davea/nm | d100b/nm | a0c/nm | Wall thicknessd/nm |
|---|---|---|---|---|---|---|
| SBA-15e | 488 | 1.24 | 8.31 | 10.23 | 11.81 | 3.50 |
| POMC0.6-800 | 654 | 0.94 | 3.84 | 9.71 | 11.21 | 7.37 |
| POMC0.8-800 | 653 | 0.94 | 3.85 | 9.71 | 11.21 | 7.36 |
| POMC1.0-800 | 703 | 1.05 | 4.11 | 9.48 | 10.95 | 6.84 |
| POMC1.2-800 | 684 | 0.98 | 4.10 | 8.73 | 10.08 | 5.98 |
| POMC1.0-800-F | 681 | 0.93 | 3.85 | 8.72 | 10.07 | 6.22 |
| POMC1.0-700 | 749 | 1.16 | 4.12 | 9.46 | 10.92 | 6.80 |
| POMC1.0-900 | 675 | 1.00 | 3.84 | 9.28 | 10.72 | 6.88 |
| POMC1.0-1000 | 676 | 0.95 | 3.85 | 8.71 | 10.06 | 6.21 |
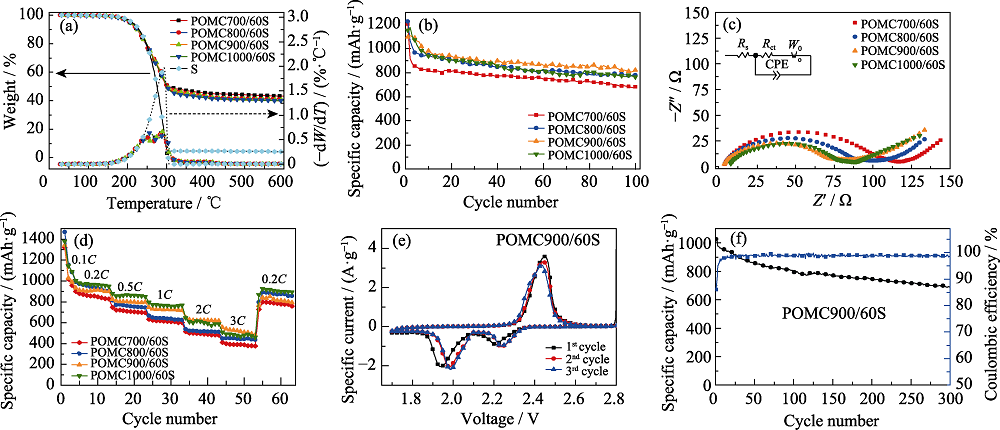
Fig. 9 (a) TG and DTG curves, (b) cycling performance at 0.2C rate, (c) EIS spectra and (d) rate performance of sample POMCt/60S, (e) CV curves and (f) long cycling performance at 0.2C rate of sample POMC900/60S
| Elemental analysis/wt% | SPa/℃ | CYb/wt% | Solubility/wt% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | Br(diff.) | H/C | TS | TI-THFS | THFI-QS | QI | ||
| 93.65 | 4.63 | 1.72 | 0.59 | 278 | 56.3 | 89.8 | 6.9 | 3.3 | 0 |
Table S1 General physical properties of methylnaphthalene pitch T290
| Elemental analysis/wt% | SPa/℃ | CYb/wt% | Solubility/wt% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | Br(diff.) | H/C | TS | TI-THFS | THFI-QS | QI | ||
| 93.65 | 4.63 | 1.72 | 0.59 | 278 | 56.3 | 89.8 | 6.9 | 3.3 | 0 |
| Pitch | Aliphatic carbons/% | Aromatic carbons/% | fa | |||
|---|---|---|---|---|---|---|
| CH3a | CH2b | Cchainc | Car1d | Car2e | ||
| T290 | 2.31 | 3.09 | 7.83 | 59.49 | 27.28 | 0.87 |
Table S2 Distributions of aliphatic and aromatic carbons in methylnaphthalene pitch T290
| Pitch | Aliphatic carbons/% | Aromatic carbons/% | fa | |||
|---|---|---|---|---|---|---|
| CH3a | CH2b | Cchainc | Car1d | Car2e | ||
| T290 | 2.31 | 3.09 | 7.83 | 59.49 | 27.28 | 0.87 |
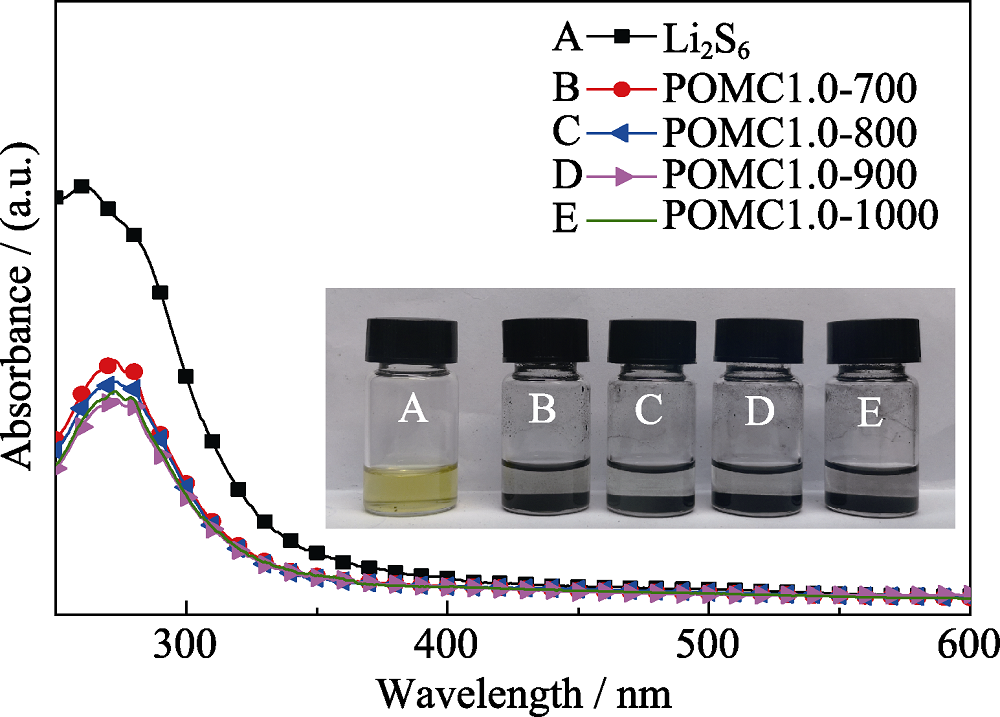
Fig. S6 UV-Vis absorption spectra of the Li2S6 solution before and after adding POMC prepared under various carbonization temperatures (inset: digital image of pure Li2S6 solution and Li2S6 solutions after adding POMC for 2 h)
| Carbon-based sulfur host | Precursor | Sulfur content /wt% | Initial capacity /(mAh·g-1) | Retention | Rate performance /(mAh·g-1) | Ref. |
|---|---|---|---|---|---|---|
| Hierarchical structure ordered mesoporous carbon | Phenolic resin | 60 | 1138 at 0.1C | ~70% after 80 cycles | 761 at 2.7C | [ |
| Core-shell structure ordered meso@microporous carbon | Sucrose | 60 | 1037 at 0.5C | ~81% after 200 cycles | 605 at 2C | [ |
| Hierarchical microporous-mesoporous carbon | Phenolic resin | 60 | 939 at 0.3C | ~78% after 150 cycles | 561 at 2C | [ |
| Mesoporous hollow carbon | Petroleum pitch | 70 | 1071 at 0.5C | ~91% after 100 cycles | 450 at 3C | [ |
| Ordered mesoporous carbon | Methylnaphthalene pitch | 60 | 1095 at 0.2C | ~78% after 100 cycles | 651 at 2C, 556 at 3C | This work |
Table S3 Comparison of electrochemical performance of POMC with similar carbon hosts for Li-S batteries
| Carbon-based sulfur host | Precursor | Sulfur content /wt% | Initial capacity /(mAh·g-1) | Retention | Rate performance /(mAh·g-1) | Ref. |
|---|---|---|---|---|---|---|
| Hierarchical structure ordered mesoporous carbon | Phenolic resin | 60 | 1138 at 0.1C | ~70% after 80 cycles | 761 at 2.7C | [ |
| Core-shell structure ordered meso@microporous carbon | Sucrose | 60 | 1037 at 0.5C | ~81% after 200 cycles | 605 at 2C | [ |
| Hierarchical microporous-mesoporous carbon | Phenolic resin | 60 | 939 at 0.3C | ~78% after 150 cycles | 561 at 2C | [ |
| Mesoporous hollow carbon | Petroleum pitch | 70 | 1071 at 0.5C | ~91% after 100 cycles | 450 at 3C | [ |
| Ordered mesoporous carbon | Methylnaphthalene pitch | 60 | 1095 at 0.2C | ~78% after 100 cycles | 651 at 2C, 556 at 3C | This work |
| [1] |
WU Z X, ZHAO D Y. Ordered mesoporous materials as adsorbents. Chem. Commun., 2011, 47(12):3332-3338.
DOI URL |
| [2] |
XU X T, TAN H B, WANG Z M, et al. Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination. Environ. Sci.: Nano, 2019, 6(3):981-989.
DOI URL |
| [3] |
NAUSHAD M, AHAMAD T, ALMASWARI B M, et al. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J., 2017, 330:1351-1360.
DOI URL |
| [4] |
RAFIEE M, KARIMI B, SHIRMOHAMMADI H. Graphitized nitrogen-doped ordered mesoporous carbon derived from ionic liquid; catalytic performance toward ORR. Electrocatalysis, 2018, 9(5):632-639.
DOI URL |
| [5] |
WANG Y, ZHANG J S, WANG X C, et al. Boron- and fluorine-containing mesoporous carbon nitride polymers: metal- free catalysts for cyclohexane oxidation. Angew. Chem. Int. Ed., 2010, 49(19):3356-3359.
DOI URL |
| [6] |
GUO B K, WANG X Q, FULVIO P F, et al. Soft-templated mesoporous carbon-carbon nanotube composites for high performance lithium-ion batteries. Adv. Mater., 2011, 23(40):4661-4666.
DOI URL |
| [7] |
LIN T Q, CHEN I, LIU F X, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science, 2015, 350(6267):1508-1513.
DOI URL |
| [8] |
SCHUSTER J, HE G, MANDLMEIER B, et al. Spherical ordered mesoporous carbon nanoparticles with high porosity for lithium- sulfur batteries. Angew. Chem. Int. Ed., 2012, 51(15):3591-3595.
DOI URL |
| [9] |
WU M B, AI P P, TAN M H, et al. Synthesis of starch-derived mesoporous carbon for electric double layer capacitor. Chem. Eng. J., 2014, 245:166-172.
DOI URL |
| [10] |
ZHI J, ZHAO W, LIU X Y, et al. Highly conductive ordered mesoporous carbon based electrodes decorated by 3D graphene and 1D silver nanowire for flexible supercapacitor. Adv. Funct. Mater., 2014, 24(14):2013-2019.
DOI URL |
| [11] |
HADOUN H, SADAOUI Z, SOUAMI N, et al. Characterization of mesoporous carbon prepared from date stems by H3PO4 chemical activation. Appl. Surf. Sci., 2013, 280:1-7.
DOI URL |
| [12] |
ZHU H W, JING Y K, PAL M, et al. Mesoporous TiO2@N-doped carbon composite nanospheres synthesized by the direct carbonization of surfactants after Sol-Gel process for superior lithium storage. Nanoscale, 2017, 9(4):1539-1546.
DOI URL |
| [13] |
HEROU S, RIBADENEYRA M C, MADHU R, et al. Ordered mesoporous carbons from lignin: a new class of biobased electrodes for supercapacitors. Green Chem., 2019, 21(3):550-559.
DOI URL |
| [14] |
BRANDIELE R, PICELLI L, PILOT R, et al. Nitrogen and sulfur doped mesoporous carbons, prepared from templating silica, as interesting material for supercapacitors. ChemistrySelect, 2017, 2(24):7082-7090.
DOI URL |
| [15] |
XU B, PENG L, WANG G Q, et al. Easy synthesis of mesoporous carbon using nano-CaCO3 as template. Carbon, 2010, 48(8):2377-2380.
DOI URL |
| [16] |
BENZIGAR M R, TALAPANENI S N, JOSEPH S, et al. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chem. Soc. Rev., 2018, 47(8):2680-2721.
DOI URL |
| [17] |
EFTEKHARI A, FAN Z Y. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front., 2017, 1(6):1001-1027.
DOI URL |
| [18] |
HONG Z Q, LI J X, ZHANG F. Synthesis of magnetically graphitic mesoporous carbon from hard templates and its application in the adsorption treatment of traditional Chinese medicine wastewater. Acta Phys.-Chim. Sin., 2013, 29(3):590-596.
DOI URL |
| [19] |
MA T Y, LIU L, YUAN Z Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev., 2013, 42(9):3977-4003.
DOI URL |
| [20] |
CAO L, KRUK M. A family of ordered mesoporous carbons derived from mesophase pitch using ordered mesoporous silicas as templates. Adsorption, 2010, 16(4):465-472.
DOI URL |
| [21] |
ZHANG X L, ZHONG C H, LIN Q L, et al. Facile fabrication of graphitic mesoporous carbon with ultrathin walls from petroleum asphalt. J. Anal. Appl. Pyrol., 2017, 126:154-157.
DOI URL |
| [22] |
ENTERRIA M, FIGUEIREDO J L. Nanostructured mesoporous carbons: tuning texture and surface chemistry. Carbon, 2016, 108:79-102.
DOI URL |
| [23] |
GAO Q, QU F Y, ZHENG W T, et al. A simple method to synthesize graphitic mesoporous carbon materials with different structures. J. Porous Mat., 2013, 20(4):983-988.
DOI URL |
| [24] |
PENG X M, HU F P, DAI H L, et al. Study of the adsorption mechanisms of ciprofloxacin antibiotics onto graphitic ordered meso- porous carbons. J. Taiwan Inst. Chem. E., 2016, 65:472-481.
DOI URL |
| [25] | XIE R L, ZONG Z M, LIU F J, et al. Nitrogen-doped porous carbon foams prepared from mesophase pitch through graphitic carbon nitride nanosheet templates. RSC Adv., 2015, 5(57):45718-45724. |
| [26] | GE C Z. Synthesis, Structure and Properties of High Performance Synthetic Pitch and Mesophase Pitch. Shanghai: PhD dissertation of East China University of Science and Technology, 2016. |
| [27] | SUN F G, WHANG J T, LONG D H, et al. A high-rate lithium-sulfur battery assisted by nitrogen-enriched mesoporous carbons decorated with ultrafine La2O3 nanoparticles. J. Mater. Chem. A, 2013, 1(42):13283-13289. |
| [28] |
HSIEH P Y, WIDEGREN J A, SLIFKA A J, et al. Direct measurement of trace polycyclic aromatic hydrocarbons in diesel fuel with 1H and 13C NMR spectroscopy: effect of PAH content on fuel lubricity. Energ. Fuel, 2015, 29(7):4289-4297.
DOI URL |
| [29] |
GE C, YANG H, MIYAWAKI J, et al. Synthesis and characterization of high-softening-point methylene-bridged pitches by visible light irradiation assisted free-radical bromination. Carbon, 2015, 95:780-788.
DOI URL |
| [30] |
CHEN S R, ZHAI Y P, XU G L, et al. Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium-sulfur battery. Electrochimica Acta, 2011, 56(26):9549-9555.
DOI URL |
| [31] |
LI Z, JIANG Y, YUAN L X, et al. A highly ordered meso@microporous carbon-supported sulfur@smaller sulfur core-shell structured cathode for Li-S batteries. ACS Nano, 2014, 8(9):9295-9303.
DOI URL |
| [32] |
LI D, HAN F, WANG S, et al. High sulfur loading cathodes fabricated using peapodlike, large pore volume mesoporous carbon for lithium-sulfur battery. ACS Appl. Mater. Inter., 2013, 5(6):2208-2213.
DOI URL |
| [33] |
NIU S Z, WU S D, LU W, et al. A one-step hard-templating method for the preparation of a hierarchical microporous- mesoporous carbon for lithium-sulfur batteries. New Carbon Mater., 2017, 32(4):289-296.
DOI URL |
| [34] |
JAYAPRAKASH N, SHEN J, MOGANTY S S, et al. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed., 2011, 50(26):5904-5908.
DOI URL |
| [1] | ZHANG Wenjun, ZHAO Xueying, LÜ Jiangwei, QU Youpeng. Progresses on Hollow Periodic Mesoporous Organosilicas: Preparation and Application in Tumor Therapy [J]. Journal of Inorganic Materials, 2022, 37(11): 1192-1202. |
| [2] | Shi-Qiang LUO, Chun-Man ZHENG, Wei-Wei SUN, Wei XIE, Jian-Huang KE, Shuang-Ke LIU, Xiao-Bin HONG, Yu-Jie LI, Jing XU. Controllable Preparation of Co-NC Nanoporous Carbon Derived from ZIF-67 for Advanced Lithium-sulfur Batteries [J]. Journal of Inorganic Materials, 2019, 34(5): 502-508. |
| [3] | LU Feng,LONG Dong-Hui,QIAO Wen-Ming,ZHAN Liang,LING Li-Cheng. Structure Control of Ordered Mesoporous Carbon Spheres Prepared from Suspension-assist Evaporationinduced Selfassembly [J]. Journal of Inorganic Materials, 2009, 24(3): 571-576. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||