
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (5): 477-484.DOI: 10.15541/jim20230532
Special Issue: 【信息功能】柔性材料(202506); 【能源环境】钙钛矿(202506); 【能源环境】太阳能电池(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
CHEN Tian1( ), LUO Yuan1, ZHU Liu2,3, GUO Xueyi1, YANG Ying1(
), LUO Yuan1, ZHU Liu2,3, GUO Xueyi1, YANG Ying1( )
)
Received:2023-11-16
Revised:2024-01-20
Published:2024-05-20
Online:2024-02-22
Contact:
YANG Ying, professor. E-mail: muyicaoyang@csu.edu.cnAbout author:CHEN Tian (1993-), female, PhD candidate. E-mail: amychen@csu.edu.cn
Supported by:CLC Number:
CHEN Tian, LUO Yuan, ZHU Liu, GUO Xueyi, YANG Ying. Organic-inorganic Co-addition to Improve Mechanical Bending and Environmental Stability of Flexible Perovskite Solar Cells[J]. Journal of Inorganic Materials, 2024, 39(5): 477-484.
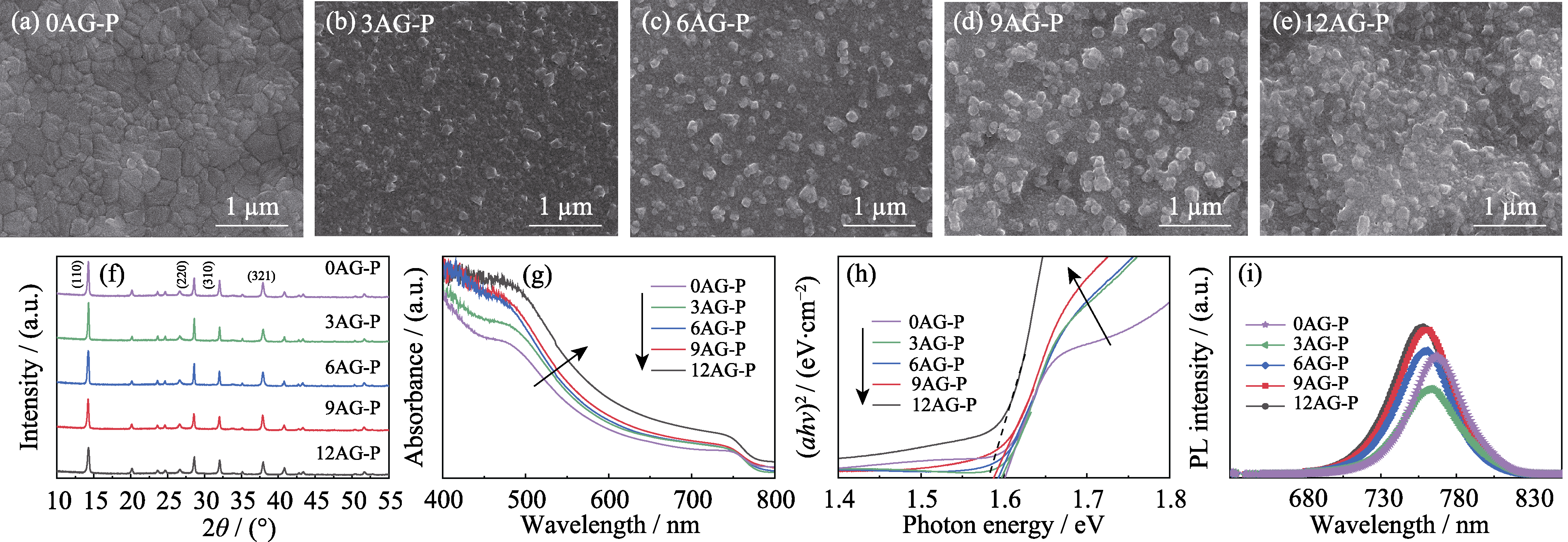
Fig. 1 Morphologies, crystal structure and optical properties of xAG-P (x=0, 3, 6, 9, 12) films deposited on ITO-PEN/SnO2 (a-e) SEM images; (f) XRD patterns; (g) UV-Vis absorption spectra; (h) Tauc plots; (i) Photoluminescence emission Colorful figures are available on website
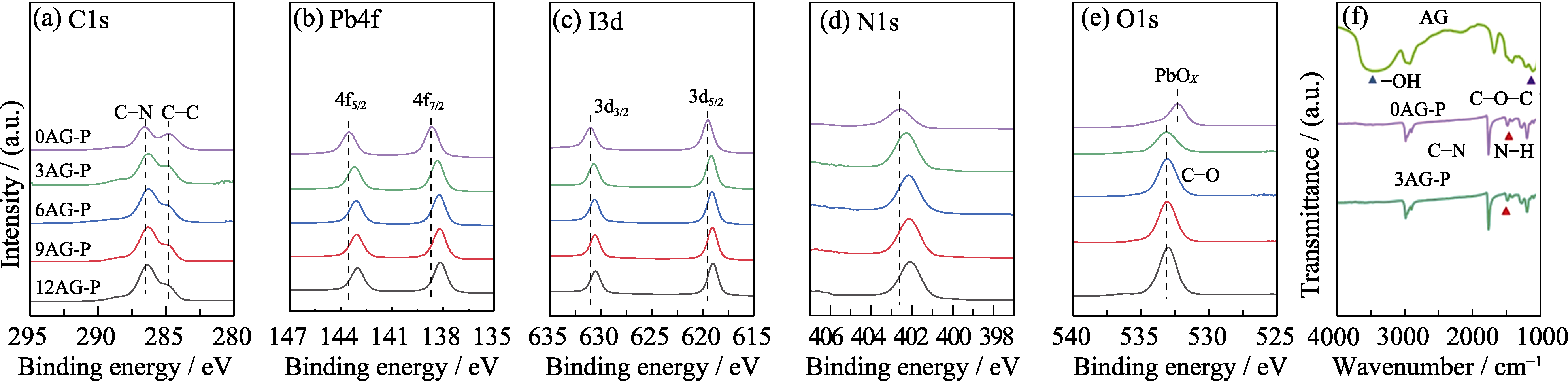
Fig. 2 (a) C1s, (b) Pb4f, (c) I3d, (d) N1s, and (e) O1s XPS spectra of xAG-P (x=0, 3, 6, 9, 12) films, and (f) FT-IR spectra of AG, 0AG-P, and 3AG-P films
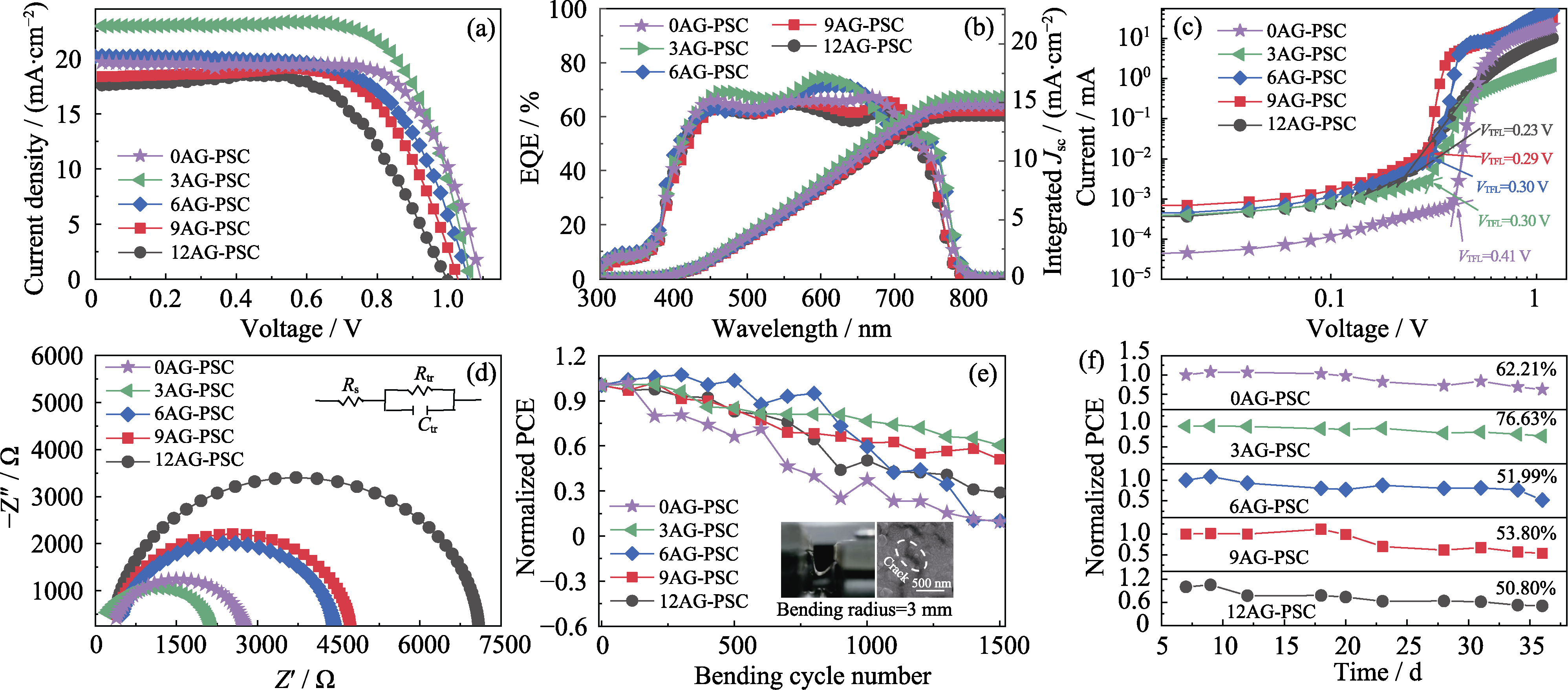
Fig. 3 Photovoltaic performance and stability of xAG-PSC (x=0, 3, 6, 9, 12) (a) J-V curves; (b) External quantum efficiency (EQE) curves; (c) I-V plots based on space charges limit current model; (d) Nyquist plots; (e) Bending stability; (f) Environmental stability Colorful figures are available on website
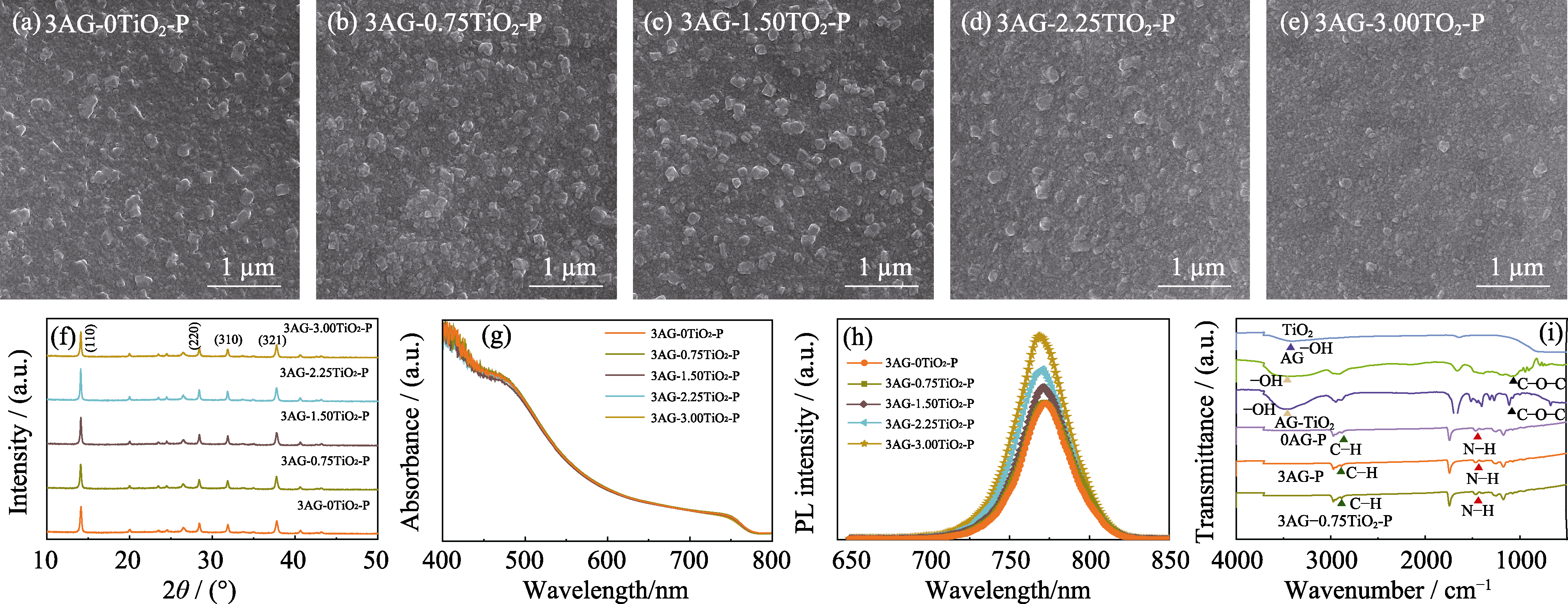
Fig. 4 Morphologies, crystal structure, and optical properties of 3AG-yTiO2-P (y=0, 0.75, 1.50, 2.25, 3.00) films deposited on ITO-PEN /SnO2 (a-e) SEM images; (f) XRD patterns; (g) UV-Vis absorption spectra; (h) Photoluminescence emission spectra; (i) FT-IR spectra of TiO2, AG, AG-TiO2, 0AG-P, 3AG-P, and 3AG-0.75TiO2-P films Colorful figures are available on website
| Sample | FWHM(110) | FWHM(220) | FWHM(310) | FWHM(321) |
|---|---|---|---|---|
| 0AG-P | 0.220 | 0.183 | 0.196 | 0.271 |
| 3AG-P | 0.165 | 0.136 | 0.149 | 0.257 |
| 6AG-P | 0.210 | 0.146 | 0.156 | 0.261 |
| 9AG-P | 0.208 | 0.199 | 0.156 | 0.260 |
| 12AG-P | 0.239 | 0.221 | 0.223 | 0.276 |
Table S1 FWHM of XRD peaks of MAPbI3 films modified with different agarose concentrations/(°)
| Sample | FWHM(110) | FWHM(220) | FWHM(310) | FWHM(321) |
|---|---|---|---|---|
| 0AG-P | 0.220 | 0.183 | 0.196 | 0.271 |
| 3AG-P | 0.165 | 0.136 | 0.149 | 0.257 |
| 6AG-P | 0.210 | 0.146 | 0.156 | 0.261 |
| 9AG-P | 0.208 | 0.199 | 0.156 | 0.260 |
| 12AG-P | 0.239 | 0.221 | 0.223 | 0.276 |
| Sample | νC−O−C/cm−1 | νO−H/cm−1 | νN−H/cm−1 |
|---|---|---|---|
| AG | 1076 | 3444 | |
| 0AG-P | 1452 | ||
| 3AG-P | 1454 |
Table S2 Stretching vibration peaks corresponding to the main functional groups of AG, 0AG-P abd 3AG-P
| Sample | νC−O−C/cm−1 | νO−H/cm−1 | νN−H/cm−1 |
|---|---|---|---|
| AG | 1076 | 3444 | |
| 0AG-P | 1452 | ||
| 3AG-P | 1454 |
| Sample | Jsc/(mA·cm-2) | Voc/V | FF/% | PCE/% |
|---|---|---|---|---|
| 0AG-PSC | 19.66 | 1.09 | 70.58 | 15.17 |
| 3AG-PSC | 22.89 | 1.06 | 71.12 | 17.30 |
| 6AG-PSC | 20.17 | 1.06 | 64.18 | 13.65 |
| 9AG-PSC | 18.31 | 1.03 | 68.45 | 12.86 |
| 12AG-PSC | 17.53 | 1.00 | 64.35 | 11.20 |
Table S3 Photoelectric performance parameters of F-PSCs based on MAPbI3 films with different agarose concentrations
| Sample | Jsc/(mA·cm-2) | Voc/V | FF/% | PCE/% |
|---|---|---|---|---|
| 0AG-PSC | 19.66 | 1.09 | 70.58 | 15.17 |
| 3AG-PSC | 22.89 | 1.06 | 71.12 | 17.30 |
| 6AG-PSC | 20.17 | 1.06 | 64.18 | 13.65 |
| 9AG-PSC | 18.31 | 1.03 | 68.45 | 12.86 |
| 12AG-PSC | 17.53 | 1.00 | 64.35 | 11.20 |
| Sample | FWHM(110) | FWHM(220) | FWHM(310) | FWHM(321) |
|---|---|---|---|---|
| 3AG-0TiO2-P | 0.165 | 0.136 | 0.149 | 0.257 |
| 3AG-0.75TiO2-P | 0.188 | 0.183 | 0.196 | 0.255 |
| 3AG-1.50TiO2-P | 0.169 | 0.193 | 0.189 | 0.263 |
| 3AG-2.25TiO2-P | 0.146 | 0.169 | 0.174 | 0.254 |
| 3AG-3.00TiO2-P | 0.161 | 0.179 | 0.189 | 0.246 |
Table S4 FWHM of XRD peaks of MAPbI3 films modified with different concentrations of TiO2/(°)
| Sample | FWHM(110) | FWHM(220) | FWHM(310) | FWHM(321) |
|---|---|---|---|---|
| 3AG-0TiO2-P | 0.165 | 0.136 | 0.149 | 0.257 |
| 3AG-0.75TiO2-P | 0.188 | 0.183 | 0.196 | 0.255 |
| 3AG-1.50TiO2-P | 0.169 | 0.193 | 0.189 | 0.263 |
| 3AG-2.25TiO2-P | 0.146 | 0.169 | 0.174 | 0.254 |
| 3AG-3.00TiO2-P | 0.161 | 0.179 | 0.189 | 0.246 |
| Sample | νC−O−C/cm−1 | νO−H/cm−1 | νC−H/cm−1 | νN−H/cm−1 |
|---|---|---|---|---|
| TiO2 | 3415 | |||
| AG | 1076 | 3444 | ||
| TiO2-AG | 1099 | 3448 | ||
| 0AG-P | 2871 | 1452 | ||
| 3AG-P | 2873 | 1454 | ||
| 3AG-0.75TiO2-P | 2873 | 1456 |
Table S5 Stretching vibration peaks corresponding to the main functional groups of TiO2, AG, TiO2-AG 0AG-P, 3AG-P and 3AG-0.75TiO2-P
| Sample | νC−O−C/cm−1 | νO−H/cm−1 | νC−H/cm−1 | νN−H/cm−1 |
|---|---|---|---|---|
| TiO2 | 3415 | |||
| AG | 1076 | 3444 | ||
| TiO2-AG | 1099 | 3448 | ||
| 0AG-P | 2871 | 1452 | ||
| 3AG-P | 2873 | 1454 | ||
| 3AG-0.75TiO2-P | 2873 | 1456 |
| [1] | The National Renewable Energy Laboratory. Best research-cell efficiency chart. (2023-07-10)[2024-01-17]. https://www.nrel.gov/pv/cell-efficiency.html. |
| [2] | FANG W, SHEN L, LI H, et al. Effect of film formation processes of NiOx mesoporous layer on performance of perovskite solar cells with carbon electrodes. J. Inorg. Mater., 2023, 38(9): 1103. |
| [3] | LUO Q, MA H, HOU Q, et al. All-carbon-electrode-based endurable flexible perovskite solar cells. Adv. Funct. Mater., 2018, 28(11): 1706777. |
| [4] | WEI J, GUO F, WANG X, et al. SnO2-in-polymer matrix for high-efficiency perovskite solar cells with improved reproducibility and stability. Adv. Mater., 2018, 30(52): 1805153. |
| [5] | LI M, ZHOU J, TAN L, et al. Multifunctional succinate additive for flexible perovskite solar cells with more than 23% power- conversion efficiency. The Innovation, 2022, 3(6): 100310. |
| [6] | ZHU C T, YANG Y, LIN F Y, et al. Electrodeposited transparent PEDOT for inverted perovskite solar cells: improved charge transport and catalytic performances. Rare Metals, 2021, 40(9): 2402. |
| [7] | YANG Y, CHEN T, PAN D, et al. MAPbI3/agarose photoactive composite for highly stable unencapsulated perovskite solar cells in humid environment. Nano Energy, 2020, 67: 104246. |
| [8] | BI D, YI C, LUO J, et al. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy, 2016, 1(10): 16142. |
| [9] | YAO Z, QU D, GUO Y, et al. Grain boundary regulation of flexible perovskite solar cells via a polymer alloy additive. Org. Electron., 2019, 70: 205. |
| [10] | LIU C, SUN J, JIANG X F, et al. A universal tactic of using Lewis-base polymer-CNTs composites as additives for high performance cm2-sized and flexible perovskite solar cells. Science China Chemistry, 2021, 64(2): 281. |
| [11] | WANG P C, GOVINDAN V, CHIANG C H, et al. Room- temperature-processed fullerene/TiO2 nanocomposite electron transporting layer for high-efficiency rigid and flexible planar perovskite solar cells. Solar RRL, 2020, 4(10): 2000247. |
| [12] | CHANG C Y, CHU C Y, HUANG Y C, et al. Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interfaces, 2015, 7(8): 4955. |
| [13] | GAO L L, LIANG L S, SONG X X, et al. Preparation of flexible perovskite solar cells by a gas pump drying method on a plastic substrate. J. Mater. Chem. A, 2016, 4(10): 3704. |
| [14] | ZHOU X, ZHANG Y, KONG W, et al. Crystallization manipulation and morphology evolution for highly efficient perovskite solar cell fabrication via hydration water induced intermediate phase formation under heat assisted spin-coating. J. Mater. Chem. A, 2018, 6(7): 3012. |
| [15] | ZONG Y, ZHOU Y, ZHANG Y, et al. Continuous grain-boundary functionalization for high-efficiency perovskite solar cells with exceptional stability. Chem, 2018, 4(6): 1404. |
| [16] | ZHAO Z, XU W, PAN G, et al. Enhancing the exciton emission of CsPbCl3 perovskite quantum dots by incorporation of Rb+ions. Mater. Res. Bull, 2019, 112: 142. |
| [17] | MEI H, WU Y, WANG C, et al. Synergistic engineering of bromine and cetyltrimethylammonium chloride molecules enabling efficient and stable flexible perovskite solar cells. J. Mater. Chem. A, 2020, 8(37): 19425. |
| [18] | SUN Q, FASSL P, BECKER‐KOCH D, et al. Role of microstructure in oxygen induced photodegradation of methylammonium lead triiodide perovskite films. Adv. Energy. Mater, 2017, 7(20): 1700977. |
| [19] | LI Y, LU K, LING X, et al. High performance planar-heterojunction perovskite solar cells using amino-based fulleropyrrolidine as the electron transporting material. J. Mater. Chem. A, 2016, 4(26): 10130. |
| [20] | YANG J, HONG Q, YUAN Z, et al. Unraveling photostability of mixed cation perovskite films in extreme environment. Adv. Opt. Mater., 2018, 6(20): 1800262. |
| [21] | LI M, YANG Y G, WANG Z K, et al. Perovskite grains embraced in a soft fullerene network make highly efficient flexible solar cells with superior mechanical stability. Adv. Mater., 2019, 31(25): 1901519. |
| [22] |
ZHANG F, XIAO C X, CHEN X H, et al. Self-seeding growth for perovskite solar cells with enhanced stability. Joule, 2019, 3(6): 1452.
DOI |
| [23] | THIESBRUMMEL J, PEÑA-CAMARGO F, BRINKMANN K O, et al. Understanding and minimizing VOC losses in all-perovskite tandem photovoltaics. Adv. Energy Mater, 2023, 13(3): 2202674. |
| [24] | WANG C, SONG Z, ZHAO D, et al. Improving performance and stability of planar perovskite solar cells through grain boundary passivation with block copolymers. Solar RRL, 2019, 3(9): 1900078. |
| [25] | NGUYEN B P, KIM G Y, JO W, et al. Trapping charges at grain boundaries and degradation of CH3NH3Pb(I1-xBrx)3 perovskite solar cells. Nanotechnology, 2017, 28(31): 315402. |
| [26] | WALI Q, IQBAL Y, PAL B, et al. Tin oxide as an emerging electron transport medium in perovskite solar cells. Sol. Energy Mater. Sol. Cells, 2018, 179: 102. |
| [27] | LEIJTENS T, EPERON G E, BARKER A J, et al. Carrier trapping and recombination: the role of defect physics in enhancing the open circuit voltage of metal halide perovskite solar cells. Energy Environ. Sci., 2016, 9(11): 3472. |
| [28] | HUANG Z, HU X, LIU C, et al. Nucleation and crystallization control via polyurethane to enhance the bendability of perovskite solar cells with excellent device performance. Adv. Funct. Mater., 2017, 27(41): 1703061. |
| [29] | CAPIGLIA C, MUSTARELLI P, QUARTARONE E, et al. Effects of nanoscale SiO2 on the thermal and transport properties of solvent-free, poly(ethylene oxide) (PEO)-based polymer electrolytes. Solid State Ionics, 1999, 118(1/2): 73. |
| [30] | SCROSATI B, CROCE F, PERSI L. Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J. Electrochem. Soc., 2000, 147(5): 1718. |
| [31] | HAN H, LIU W, ZHANG J, et al. A hybrid poly(ethylene oxide)/poly(vinylidene fluoride)/TiO2 nanoparticle solid‐state redox electrolyte for dye-sensitized nanocrystalline solar cells. Adv. Funct. Mater., 2005, 15(12): 1940. |
| [1] | PAN Zesheng, YOU Yaping, ZHENG Ya, CHEN Haijie, WANG Lianjun, JIANG Wan. Stability of Phosphors for White LED Excitable by Violet Light [J]. Journal of Inorganic Materials, 2025, 40(3): 314-322. |
| [2] | WANG Yu, XIONG Hao, HUANG Xiaokun, JIANG Linqin, WU Bo, LI Jiansheng, YANG Aijun. Regulation of Low-dose Stannous Iso-octanoate for Two-step Prepared Sn-Pb Alloyed Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(12): 1339-1347. |
| [3] | ZHAO Hui-Yue, WANG Xiao-Dong, FENG Jian-Bin, LIU Yuan, HUANG Ji-Chen, SHEN Jun. Environmental Stable SiO2 Antireflective Coating Modified via NH3/HTMS Vapor Phase Treatment [J]. Journal of Inorganic Materials, 2018, 33(11): 1219-1224. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||