
Journal of Inorganic Materials ›› 2026, Vol. 41 ›› Issue (3): 385-392.DOI: 10.15541/jim20250236
• RESEARCH ARTICLE • Previous Articles Next Articles
TANG Yifan1( ), HUANG Zeai1,2(
), HUANG Zeai1,2( ), ZHANG Ruiyang1,2, ZHAN Junjie1, CHEN Guoxing3, YANG Mingkai1, LIU Tong1, CHEN Hongji1, ZHOU Ying1,2(
), ZHANG Ruiyang1,2, ZHAN Junjie1, CHEN Guoxing3, YANG Mingkai1, LIU Tong1, CHEN Hongji1, ZHOU Ying1,2( )
)
Received:2025-06-04
Revised:2025-08-26
Published:2025-09-11
Online:2025-09-11
Contact:
HUANG Zeai, associate professor. E-mail: zeai.huang@swpu.edu.cn;About author:TANG Yifan (2001-), female, Master candidate. E-mail: 15309939516@139.com
Supported by:CLC Number:
TANG Yifan, HUANG Zeai, ZHANG Ruiyang, ZHAN Junjie, CHEN Guoxing, YANG Mingkai, LIU Tong, CHEN Hongji, ZHOU Ying. Morphology Control of Carbon Products from Catalytic Pyrolysis of Methane with Different Concentrations in Molten Salt[J]. Journal of Inorganic Materials, 2026, 41(3): 385-392.
| Medicine | PXCl/% | MX/(g·mol-1) | MXCl/(g·mol-1) |
|---|---|---|---|
| MnCl2·4H2O | 99 | 54.9 | 197.9 |
| CaCl2 | 96 | 40.1 | 111.0 |
| NiCl2·6H2O | 98 | 58.7 | 237.7 |
| CuCl2 | 98 | 63.6 | 134.5 |
| LiCl | 99 | 6.9 | 42.4 |
Table 1 Calculation of drug dosage for metal salt screening experiment
| Medicine | PXCl/% | MX/(g·mol-1) | MXCl/(g·mol-1) |
|---|---|---|---|
| MnCl2·4H2O | 99 | 54.9 | 197.9 |
| CaCl2 | 96 | 40.1 | 111.0 |
| NiCl2·6H2O | 98 | 58.7 | 237.7 |
| CuCl2 | 98 | 63.6 | 134.5 |
| LiCl | 99 | 6.9 | 42.4 |
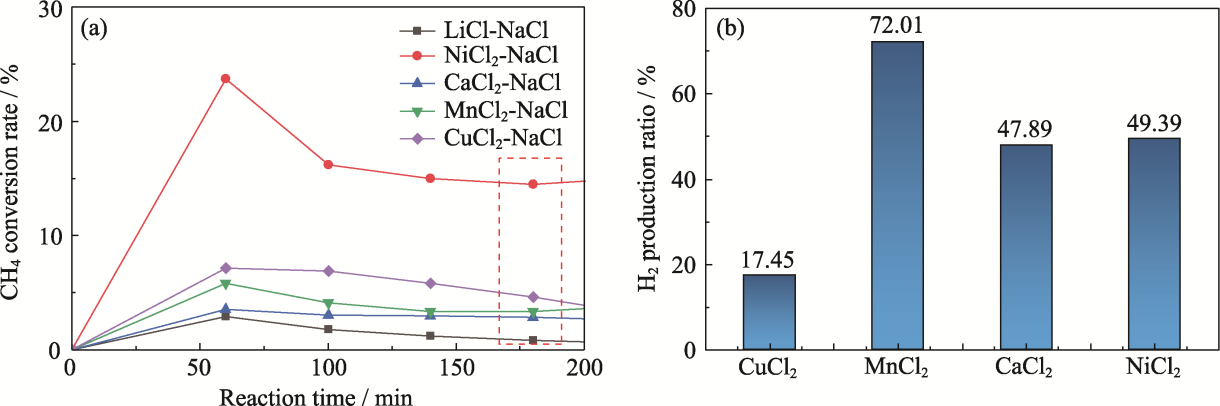
Fig. 2 Results on catalytic pyrolysis of metal chlorides (a) Time variation curves of CH4 conversion rate; (b) H2 production ratio in the product after 3 h of reaction
| Molten metal | CH4 conversion rate/% | Carbon yield/ (mg·min-1) |
|---|---|---|
| CuCl2-NaCl | 4.6 | 0.5 |
| MnCl2-NaCl | 3.3 | 0.4 |
| CaCl2-NaCl | 2.8 | 0.3 |
| NiCl2-NaCl | 14.6 | 1.6 |
| LiCl-NaCl | 0.9 | 0.1 |
Table 2 Experimental data on catalytic pyrolysis activity of metal chlorides
| Molten metal | CH4 conversion rate/% | Carbon yield/ (mg·min-1) |
|---|---|---|
| CuCl2-NaCl | 4.6 | 0.5 |
| MnCl2-NaCl | 3.3 | 0.4 |
| CaCl2-NaCl | 2.8 | 0.3 |
| NiCl2-NaCl | 14.6 | 1.6 |
| LiCl-NaCl | 0.9 | 0.1 |
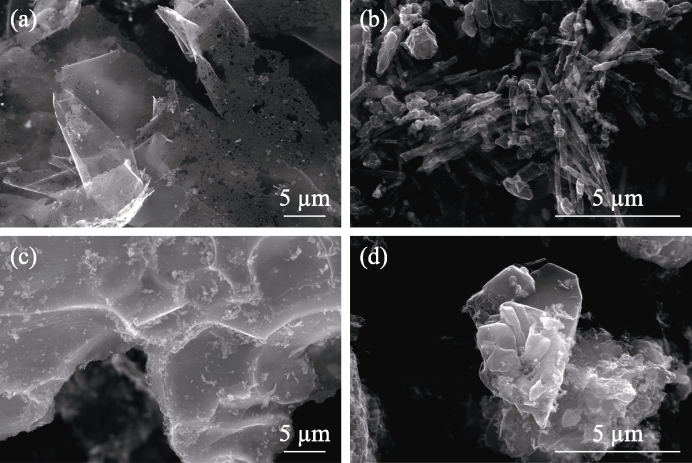
Fig. 3 SEM images of carbon products from catalytic pyrolysis of molten metal chlorides (a) CuCl2-NaCl; (b) MnCl2-NaCl; (c) CaCl2-NaCl; (d) NiCl2-NaCl
| [1] | 何展军, 黄敏, 林铁军, 等. 光热催化甲烷干重整研究进展. 物理化学学报, 2023, 39(9): 28. |
| [2] | KOSTEN S, BODMER P. Editorial for the virtual special issue: the role of plants in regulating aquatic methane fluxes. Aquatic Botany, 2024, 193: 103775. |
| [3] | ZHU X C, DI D R, MA M G, et al. Stable isotopes in greenhouse gases from soil: a review of theory and application. Atmosphere, 2019, 10(7): 377. |
| [4] | GU A L, ZHOU S, XU S Q, et al. Energy industry methane emissions trajectory analysis in China until 2050. Atmosphere, 2022, 13(12): 1989. |
| [5] | SAUNOIS M, BOUSQUET P, POULTER B, et al. The global methane budget 2000-2017. Earth System Science Data, 2020, 12(4): 1561. |
| [6] | 胡婉玲, 黄玛兰, 王红玲. 低碳背景下畜牧业甲烷排放现状与减排策略研究. 华中农业大学学报(自然科学版), 2022, 41(3): 115. |
| [7] | GAO J L, GUAN C H, ZHANG B, et al. Decreasing methane emissions from China’s coal mining with rebounded coal production. Environmental Research Letters, 2021, 16(12): 124037. |
| [8] | GEUM S, PARK H, CHOI H, et al. Identifying emission sources of CH4 in East Asia based on in situ observations of atmospheric δ13C-CH4 and C2H6. Science of the Total Environment, 2024, 908: 168433. |
| [9] | FUJITA R, GRAVEN H, ZAZZERI G, et al. Global fossil methane emissions constrained by multi-isotopic atmospheric methane histories. Journal of Geophysical Research: Atmospheres, 2025, 130(5): e2024JD041266. |
| [10] | XIAO C L, FU B H, SHUI H Q, et al. Detecting the sources of methane emission from oil shale mining and processing using airborne hyperspectral data. Remote Sensing, 2020, 12(3): 537. |
| [11] | KNAPP J R, LAUR G L, VADAS P A, et al. Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. Journal of Dairy Science, 2014, 97(6): 3231. |
| [12] | 刘桂凤, 皮希宇, 王栓林, 等. 瓦斯抽采与利用技术的现状分析. 煤炭与化工, 2015, 38(3): 5. |
| [13] | 李国富, 李超, 霍春秀, 等. 山西重点煤矿区瓦斯梯级利用关键技术与工程示范. 煤田地质与勘探, 2022, 50(9): 42. |
| [14] | 高鹏飞. 乏风瓦斯提浓利用技术现状及展望. 矿业安全与环保, 2017, 44(3): 95. |
| [15] | 张博, 郭金玲, 高俊莲, 等. 我国甲烷排放控制的中长期挑战与应对. 中国工程科学, 2024, 26(2): 185. |
| [16] | BLUMBERG T, MOROSUK T, TSATSARONIS G. A comparative exergoeconomic evaluation of the synthesis routes for methanol production from natural gas. Applied Sciences, 2017, 7(12): 1213. |
| [17] | 黄兴, 赵博宇, LOUGOU B G, 等. 甲烷水蒸气重整制氢研究进展. 石油与天然气化工, 2022, 51(1): 53. |
| [18] | 罗化峰, 李通达, 乔元栋, 等. Ca(OH)2对煤基活性炭及其催化甲烷裂解制氢的影响. 现代化工, 2021, 41(4): 162. |
| [19] | 杨丽, 刘帅, 辛春梅, 等. 炭黑负载增加活性炭缺陷位点催化甲烷裂解制氢机理研究. 煤炭科学技术, 2024, 52(3): 300. |
| [20] | ZHANG K K, HUANG Z A, YANG M K, et al. Recent progress in melt pyrolysis: fabrication and applications of high-value carbon materials from abundant sources. SusMat, 2023, 3(5): 558. |
| [21] | 李雅欣, 何阳东, 刘韬, 等. 甲烷裂解制氢工艺研究进展及技术经济性对比分析. 石油与天然气化工, 2022, 51(3): 38. |
| [22] | 赵西, 王丹, 丁桐, 等. 甲烷等离子体法制氢气和碳材料研究进展. 石油与天然气化工, 2023, 52(1): 40. |
| [23] | CHAN Y H, CHAN Z P, LOCK S S M, et al. Thermal pyrolysis conversion of methane to hydrogen (H2): a review on process parameters, reaction kinetics and techno-economic analysis. Chinese Chemical Letters, 2024, 35(8): 109329. |
| [24] | 何阳东, 常宏岗, 王丹, 等. 熔融金属法甲烷裂解制氢和碳材料研究进展. 化工进展, 2023, 42(3): 1270. |
| [25] | 覃莉, 何阳东, 陈昌武, 等. 熔融法天然气裂解制石墨烯工艺及应用研究. 石油与天然气化工, 2024, 53(5): 25. |
| [26] | 覃莉, 何阳东, 曾正荣, 等. 天然气氢炭联产工艺研究进展. 石油与天然气化工, 2023, 52(4): 48. |
| [27] | KORÁNYI T I, NÉMETH M, BECK A, et al. Recent advances in methane pyrolysis: turquoise hydrogen with solid carbon production. Energies, 2022, 15(17): 6342. |
| [28] | GUNARAYU M R, ABDUL PATAH M F, ASHRI WAN DAUD W M. Advancements in methane pyrolysis: a comprehensive review of parameters and molten catalysts in bubble column reactors. Renewable and Sustainable Energy Reviews, 2025, 210: 115197. |
| [29] | BAE D, KIM Y, KO E H, et al. Methane pyrolysis and carbon formation mechanisms in molten manganese chloride mixtures. Applied Energy, 2023, 336: 120810. |
| [30] | KANG D, RAHIMI N, GORDON M J, et al. Catalytic methane pyrolysis in molten MnCl2-KCl. Applied Catalysis B: Environmental, 2019, 254: 659. |
| [31] | KANG D, PALMER C, MANNINI D, et al. Catalytic methane pyrolysis in molten alkali chloride salts containing iron. ACS Catalysis, 2020, 10(13): 7032. |
| [1] | YANG Mingkai, HUANG Zeai, ZHOU Yunxiao, LIU Tong, ZHANG Kuikui, TAN Hao, LIU Mengying, ZHAN Junjie, CHEN Guoxing, ZHOU Ying. Co-production of Few-layer Graphene and Hydrogen from Methane Pyrolysis Based on Cu and Metal Oxide-KCl Molten Medium [J]. Journal of Inorganic Materials, 2025, 40(5): 473-480. |
| [2] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [3] | WEN Hai-Meng, SONG Jun, WANG Chong-Qing, ZHOU Yu, WANG Jun. Directly Synthesis of ZSM-22 Particles by Adding Polyurethane Foam in Ionic Liquid-directed Dry-gel-conversion [J]. Journal of Inorganic Materials, 2015, 30(6): 615-620. |
| [4] | ZHU Chun-Ye,XIE Zi-Li,GUO Kun-Min. Morphology Control of Vapor Grown Carbon Nanofibers [J]. Journal of Inorganic Materials, 2004, 19(3): 599-604. |
| [5] | WEN Yan,XIANG Lan,JIN Yong. Preparation of Plate-like Calcium Carbonate [J]. Journal of Inorganic Materials, 2002, 17(6): 1315-1320. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||