
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (11): 1265-1274.DOI: 10.15541/jim20240074
Special Issue: 【能源环境】氢能材料(202506); 【结构材料】高熵陶瓷(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
ZHANG Wenyu1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), YUE Quanxin1,2,3, HUANG Yarong1, ZHANG Guofang1, GUAN Lili1,2
), YUE Quanxin1,2,3, HUANG Yarong1, ZHANG Guofang1, GUAN Lili1,2
Received:2024-02-21
Revised:2024-05-23
Published:2024-11-20
Online:2024-06-24
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:ZHANG Wenyu (1997-), male, Master candidate. E-mail: zhangwenyu529@qq.com
Supported by:CLC Number:
ZHANG Wenyu, GUO Ruihua, YUE Quanxin, HUANG Yarong, ZHANG Guofang, GUAN Lili. High-entropy Phosphide Bifunctional Catalyst: Preparation and Performance of Efficient Water Splitting[J]. Journal of Inorganic Materials, 2024, 39(11): 1265-1274.
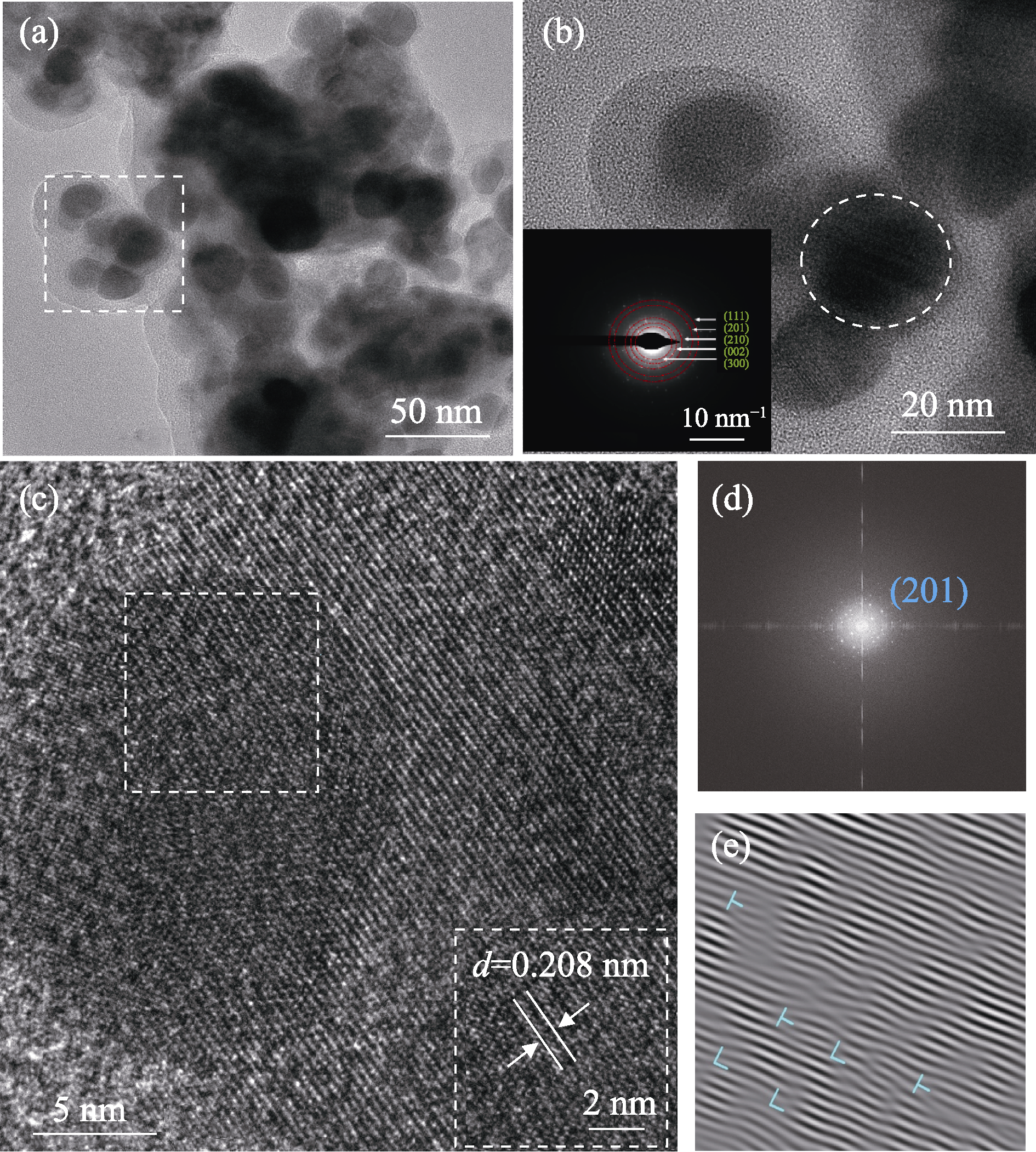
Fig. 3 TEM and high-resolution TEM (HRTEM) images of FeCoNiMoCeP/C (a) TEM image; (b) HRTEM image and selected-area electron diffraction (SAED) image (inset) of the square region in (a); (c) HRTEM image of the circular region in (b); (d) Fast Fourier transform (FFT) and (e) inverse fast Fourier transform (IFFT) images of the square region in (c)
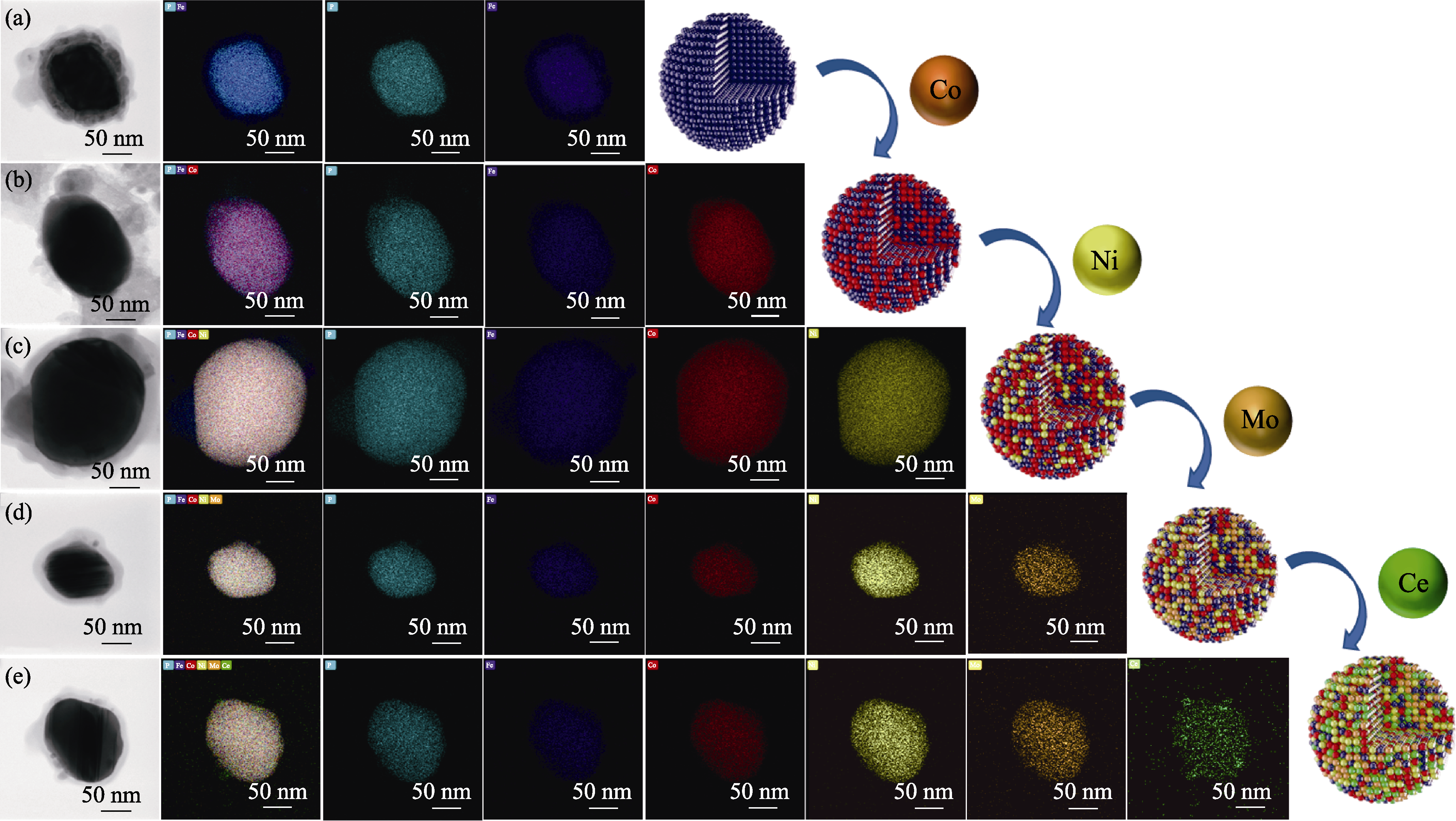
Fig. 4 High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images and corresponding EDS elemental mappings of different samples (a) FeP/C; (b) FeCoP/C; (c) FeCoNiP/C; (d) FeCoNiMoP/C; (e) FeCoNiMoCeP/C
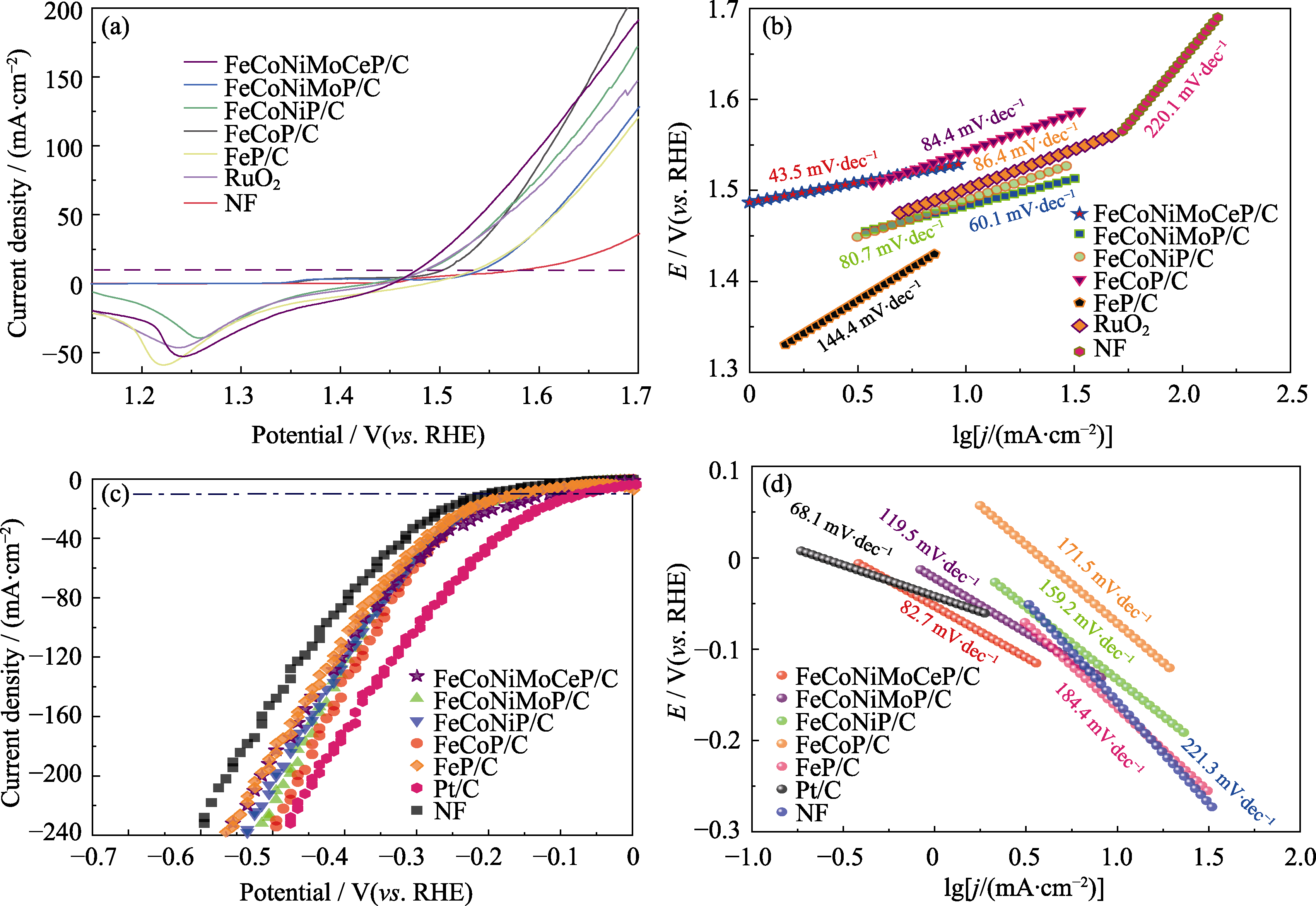
Fig. 6 OER and HER performance of different catalysts (a) OER LSV curves; (b) OER Tafel plots; (c) HER LSV curves; (d) HER Tafel plots. Colorful figures are available on website
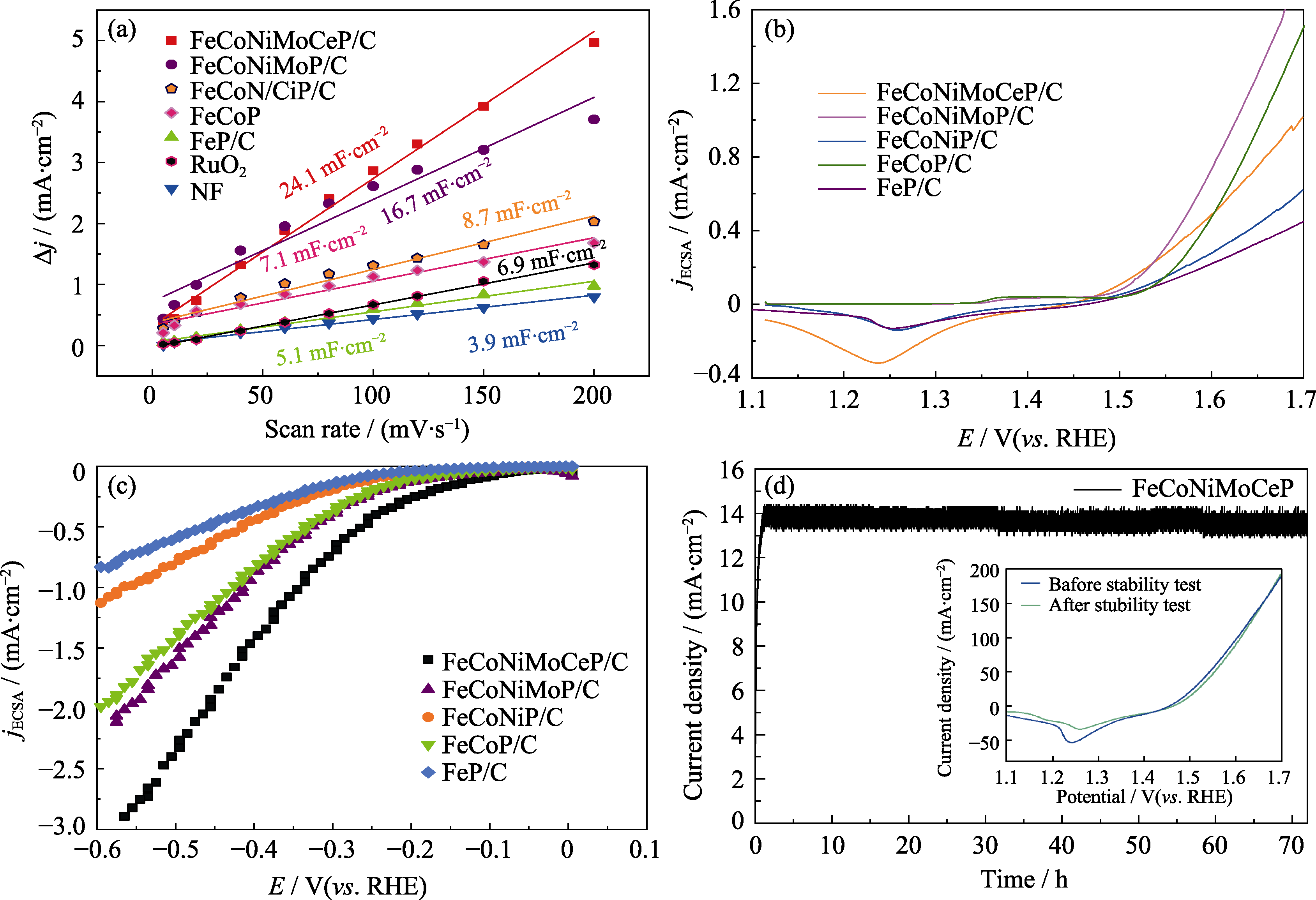
Fig. 7 (a) Double-layer capacitance (Cdl) curves, (b) SECSA-normalized OER LSV curves, and (c) SECSA-normalized HER LSV curves for different catalysts; (d) Current-time (I-t) curve of FeCoNiMoCeP/C catalyst Inset in (d): LSV curves of FeCoNiMoCeP/C before and after 72 h stability test at 1.5 V. Colorful figures are available on website
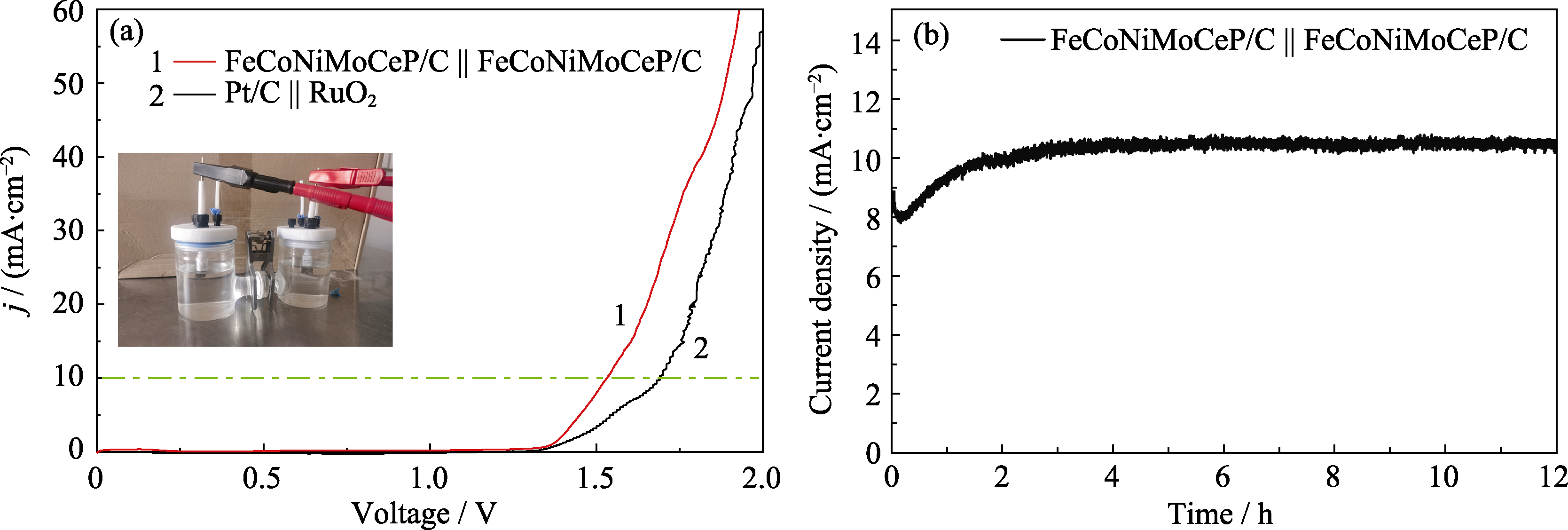
Fig. 8 Performance analysis of FeCoNiMoCeP/C || FeCoNiMoCeP/C for overall water splitting (a) LSV curves of FeCoNiMoCeP/C || FeCoNiMoCeP/C and Pt/C || RuO2 with inset showing photograph of overall water splitting equipment with two electrodes; (b) I-t curve of FeCoNiMoCeP/C || FeCoNiMoCeP/C
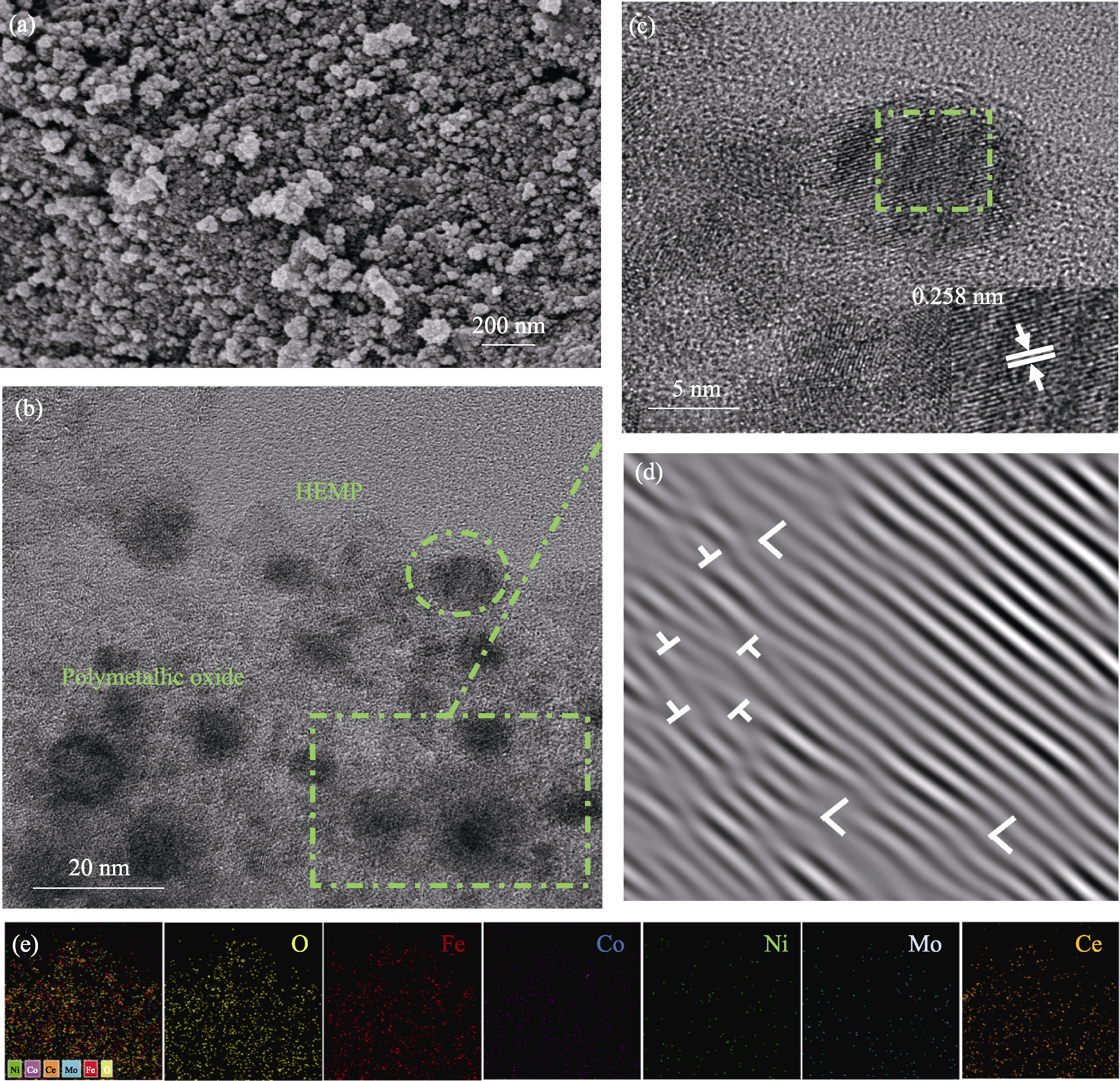
Fig. S4 Structure morphology and EDS elemental mappings of FeCoNiMoCeP/C after stability test for 72 h (a) SEM image; (b) HRTEM image; (c) Crystallographic planes and interplanar spacing marked by dashed boxes in (b); (d) IFFT pattern corresponding to the selected area marked by the dashed array in (c); (e) Elemental mappings
| Sample | Electrolyte solvent | η10 OER/ mV | η10 HER/ mV | OER Tafel slope/ (mV·dec-1) | HER Tafel slope/ (mV·dec-1) | η10 overall water splitting/V | Stability/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeCoNiMoCeP/C | 1.0 mol·L-1 KOH | 240 | 119 | 63.4 | 82.7 | 1.53 | 72 | This work |
| NiCoFeMnCrP | 1.0 mol·L-1 KOH | 270 | 220 | 52.5 | 94.5 | 1.55 | 24 | [ |
| FeNiCoMnCu@CNT | 1.0 mol·L-1 KOH+Sea water | 380 | 290 | 130.0 | 171.0 | 1.60 | 40 | [ |
| CoNiCuMnAl@C 2 h | 1.0 mol·L-1 KOH+Sea water | 290 | - | 66.8 | - | 1.54 | 30 | [ |
| CoCrFeNiMo | 1.0 mol·L-1 KOH | 270 | 157 | 62.5 | 46.7 | 1.86 | 22 | [ |
| CoZnCdCuMnS@CF | 1.0 mol·L-1 KOH | 220 | 173 | 69.8 | 98.5 | 1.63 | 70 | [ |
| La-HEO@NiFeOOH(1 : 3) | 1.0 mol·L-1 KOH | 262 | - | 38.0 | - | 1.57 | 30 | [ |
Table S1 OER, HER, and overall water splitting performance of the FeCoNiMoCeP/C and catalysts in literature at 10 mA·cm-2
| Sample | Electrolyte solvent | η10 OER/ mV | η10 HER/ mV | OER Tafel slope/ (mV·dec-1) | HER Tafel slope/ (mV·dec-1) | η10 overall water splitting/V | Stability/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeCoNiMoCeP/C | 1.0 mol·L-1 KOH | 240 | 119 | 63.4 | 82.7 | 1.53 | 72 | This work |
| NiCoFeMnCrP | 1.0 mol·L-1 KOH | 270 | 220 | 52.5 | 94.5 | 1.55 | 24 | [ |
| FeNiCoMnCu@CNT | 1.0 mol·L-1 KOH+Sea water | 380 | 290 | 130.0 | 171.0 | 1.60 | 40 | [ |
| CoNiCuMnAl@C 2 h | 1.0 mol·L-1 KOH+Sea water | 290 | - | 66.8 | - | 1.54 | 30 | [ |
| CoCrFeNiMo | 1.0 mol·L-1 KOH | 270 | 157 | 62.5 | 46.7 | 1.86 | 22 | [ |
| CoZnCdCuMnS@CF | 1.0 mol·L-1 KOH | 220 | 173 | 69.8 | 98.5 | 1.63 | 70 | [ |
| La-HEO@NiFeOOH(1 : 3) | 1.0 mol·L-1 KOH | 262 | - | 38.0 | - | 1.57 | 30 | [ |
| [1] | STAFFELL I, SCAMMAN D, ABAD V A, et al. The role of hydrogen and fuel cells in the global energy system. Energy & Environmental Science, 2019, 12(2): 463. |
| [2] |
KIBSGAARD J, CHORKENDORFF I. Considerations for the scaling-up of water splitting catalysts. Nature Energy, 2019, 4(6): 430.
DOI |
| [3] | ANWAR S, KHAN F, ZHANG Y, et al. Recent development in electrocatalysts for hydrogen production through water electrolysis. International Journal of Hydrogen Energy, 2021, 46(63): 32284. |
| [4] |
BATCHELOR T A A, PEDERSEN J K, WINTHER S H, et al. High-entropy alloys as a discovery platform for electrocatalysis. Joule, 2019, 3(3): 834.
DOI |
| [5] | CHEN P C, LIU M, DU J S, et al. Interface and heterostructure design in polyelemental nanoparticles. Science, 2019, 363(6430): 959. |
| [6] |
YAO Y, HUANG Z, XIE P, et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science, 2018, 359(6383): 1489.
DOI PMID |
| [7] | YAN L, CAO L, DAI P, et al. Metal-organic frameworks derived nanotube of nickel-cobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Advanced Functional Materials, 2017, 27(40): 1703455. |
| [8] | ZHAO Y, JIA N, WU X R, et al. Rhodium phosphide ultrathin nanosheets for hydrazine oxidation boosted electrochemical water splitting. Applied Catalysis B: Environmental, 2020, 270: 118880. |
| [9] | YANG X, GUO R, CAI R, et al. Engineering high-entropy materials for electrocatalytic water splitting. International Journal of Hydrogen Energy, 2022, 47(28): 13561. |
| [10] | SUN M, LIU H, QU J, et al. Earth-rich transition metal phosphide for energy conversion and storage. Advanced Energy Materials, 2016, 6(13): 1600087. |
| [11] | WU R, XIAO B, GAO Q, et al. A Janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting. Angewandte Chemie International Edition, 2018, 57(47): 15445. |
| [12] | TIAN L, YAN X, CHEN X. Electrochemical activity of iron phosphide nanoparticles in hydrogen evolution reaction. ACS Catalysis, 2016, 6(8): 5441. |
| [13] | LAI D, KANG Q, GAO F, et al. High-entropy effect of a metal phosphide on enhanced overall water splitting performance. Journal of Materials Chemistry A, 2021, 9(33): 17913. |
| [14] | WANG X, DONG Q, QIAO H, et al. Continuous synthesis of hollow high-entropy nanoparticles for energy and catalysis applications. Advanced Materials, 2020, 32(46): 2002853. |
| [15] | JING X X, CHEN B Q, ZHAI J X. Ni-Co-B-RE (Sm, Dy, Tb) Composite electrodes: preparation by chemical deposition method and their electrocatalytic hydrogen evolution performance. Journal of Inorganic Materials, 2024, 39(5): 467. |
| [16] | SIVANANTHAM A, LEE H, HWANG S W, et al. Preparation, electrical and electrochemical characterizations of CuCoNiFeMn high-entropy-alloy for overall water splitting at neutral-pH. Journal of Materials Chemistry A, 2021, 9(31): 16841. |
| [17] | LI M, MEI S, ZHENG Y, et al. High-entropy oxides as photocatalysts for organic conversion. Chemical Communications, 2023, 59(90): 13478. |
| [18] | HUANG K, ZHANG B, WU J, et al. Exploring the impact of atomic lattice deformation on oxygen evolution reactions based on a sub-5 nm pure face-centred cubic high-entropy alloy electrocatalyst. Journal of Materials Chemistry A, 2020, 8(24): 11938. |
| [19] | HUANG K, PENG D, YAO Z, et al. Cathodic plasma driven self- assembly of HEAs dendrites by pure single FCC FeCoNiMnCu nanoparticles as high efficient electrocatalysts for OER. Chemical Engineering Journal, 2021, 425: 131533. |
| [20] |
ROMERO J, VARELA M, ASSEBBAN M, et al. Insights into the formation of metal carbon nanocomposites for energy storage using hybrid NiFe layered double hydroxides as precursors. Chemical Science, 2020, 11(29): 7626.
DOI PMID |
| [21] | SONG H, WU M, TANG Z, et al. Single atom ruthenium-doped CoP/CDs nanosheets via splicing of carbon-dots for robust hydrogen production. Angewandte Chemie International Edition, 2021, 60(13): 7234. |
| [22] |
LI D, GUO Z, ZHAO R, et al. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for all-pH hydrogen evolution reaction and overall water splitting. Journal of Colloid and Interface Science, 2024, 653: 1725.
DOI PMID |
| [23] | WANG X, ZHANG J, WANG P, et al. Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction. Energy & Environmental Science, 2023, 16(11): 5500. |
| [24] | WANG Y, TAO S, LIN H, et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy, 2021, 81: 105606. |
| [25] | JIN L, WANG Q, WANG K, et al. Engineering NiMoO4/NiFe LDH/rGO multicomponent nanosheets toward enhanced electrocatalytic oxygen evolution reaction. Dalton Transactions, 2022, 51(16): 6448. |
| [26] | LV Q, YAO B, ZHANG W, et al. Controlled direct electrodeposition of crystalline NiFe/amorphous NiFe-(oxy)hydroxide on NiMo alloy as a highly efficient bifunctional electrocatalyst for overall water splitting. Chemical Engineering Journal, 2022, 446: 137420. |
| [27] | LUO M, ZHAO Z, ZHANG Y, et al. PdMo bimetallene for oxygen reduction catalysis. Nature, 2019, 574(7776): 81. |
| [28] | XU H, WANG B, SHAN C, et al. Ce-doped NiFe-layered double hydroxide ultrathin nanosheets/nanocarbon hierarchical nanocomposite as an efficient oxygen evolution catalyst. ACS Applied Materials & Interfaces, 2018, 10(7): 6336. |
| [29] | HA D H, HAN B, RISCH M, et al. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy, 2016, 29: 37. |
| [30] | TAO H B, XU Y, HUANG X, et al. A general method to probe oxygen evolution intermediates at operating conditions. Joule, 2019, 3(6): 1498. |
| [31] | ZHANG Y, ZHU X, ZHANG G, et al. Rational catalyst design for oxygen evolution under acidic conditions: strategies toward enhanced electrocatalytic performance. Journal of Materials Chemistry A, 2021, 9(10): 5890. |
| [32] | BIAN H, QI P, XIE G, et al. HEA-NiFeCuCoCe/NF through ultra-fast electrochemical self-reconstruction with high catalytic activity and corrosion resistance for seawater electrolysis. Chemical Engineering Journal, 2023, 477: 147286. |
| [33] | CONG Y, CHEN X, MEI Y, et al. CeO2 decorated bimetallic phosphide nanowire arrays for enhanced oxygen evolution reaction electrocatalysis via interface engineering. Dalton Transactions, 2022, 51(7): 2923. |
| [34] | WANG A J, CHEN J, ZHANG P F, et al. Relation between NiMo(O) phase structures and hydrogen evolution activities of water electrolysis. Acta Physico-Chimica Sinica, 2023, 39(4): 2301023. |
| [35] |
ZHANG Q, LIAN K, LIU Q, et al. High entropy alloy nanoparticles as efficient catalysts for alkaline overall seawater splitting and Zn-air batteries. Journal of Colloid and Interface Science, 2023, 646: 844.
DOI PMID |
| [36] | WANG S, HUO W, FANG F, et al. High entropy alloy/C nanoparticles derived from polymetallic MOF as promising electrocatalysts for alkaline oxygen evolution reaction. Chemical Engineering Journal, 2022, 429: 132410. |
| [37] | HUO X, ZUO X, WANG X, et al. High entropy alloy CoCrFeNiMo reinforced electrocatalytic performance for high-efficient electrocatalytic water splitting. Chemistry-An Asian Journal, 2023, 18(15): e202300456. |
| [38] | LEI Y, ZHANG L, XU W, et al. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Research, 2022, 15(7): 6054. |
| [39] | WANG Z, HAN S, ZHANG Y, et al. Decorated NiFeOOH on high entropy perovskite oxide by interface engineering for efficient oxygen evolution and overall water splitting. Fuel, 2024, 357: 129946. |
| [1] | CHEN Libo, SHENG Ying, WU Ming, SONG Jiling, JIAN Jian, SONG Erhong. Na and O Co-doped Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution [J]. Journal of Inorganic Materials, 2025, 40(5): 552-562. |
| [2] | YUE Quanxin, GUO Ruihua, WANG Ruifen, AN Shengli, ZHANG Guofang, GUAN Lili. 3D Core-shell Structured NiMoO4@CoFe-LDH Nanorods: Performance of Efficient Oxygen Evolution Reaction and Overall Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1254-1264. |
| [3] | SUN Qiangqiang, CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue. Amorphous Vanadium Oxide Loaded by Metallic Nickel-copper towards High-efficiency Electrocatalyzing Hydrogen Production [J]. Journal of Inorganic Materials, 2023, 38(6): 647-655. |
| [4] | WANG Ping,LI Xinyu,SHI Zhanling,LI Haitao. Synergistic Effect of Ag and Ag2O on Photocatalytic H2-evolution Performance of TiO2 [J]. Journal of Inorganic Materials, 2020, 35(7): 781-788. |
| [5] | ZHAO Cai-Xia,ZHANG Wei-De. Preparation and Antibacterial Properties of Titanium (Ⅳ) and Zinc (Ⅱ) Co-doped Nanohydroxyapatite [J]. Journal of Inorganic Materials, 2009, 24(6): 1243-1248. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||