
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (11): 1201-1211.DOI: 10.15541/jim20240518
Special Issue: 【能源环境】金属有机框架材料MOF(202510)
• RESEARCH ARTICLE • Previous Articles Next Articles
LI Yongfeng1,2( ), GU Yuping2,3,4, SHI Guangzhao2,3,4, HU Jiulin2,3,4, LEI Meng2,3,4, PENG Hui1,5(
), GU Yuping2,3,4, SHI Guangzhao2,3,4, HU Jiulin2,3,4, LEI Meng2,3,4, PENG Hui1,5( ), ZENG Yuping2,3, LI Chilin2,3,4(
), ZENG Yuping2,3, LI Chilin2,3,4( )
)
Received:2024-12-16
Revised:2025-03-07
Published:2025-11-20
Online:2025-03-24
Contact:
LI Chilin, professor. E-mail: chilinli@mail.sic.ac.cn;About author:LI Yongfeng (1999-), male, Master candidate. E-mail: 51254700112@stu.ecnu.edu.cn
Supported by:CLC Number:
LI Yongfeng, GU Yuping, SHI Guangzhao, HU Jiulin, LEI Meng, PENG Hui, ZENG Yuping, LI Chilin. Interface Regulation of Electrochemical Potential in NASICON-type Ceramic Solid-state Batteries[J]. Journal of Inorganic Materials, 2025, 40(11): 1201-1211.
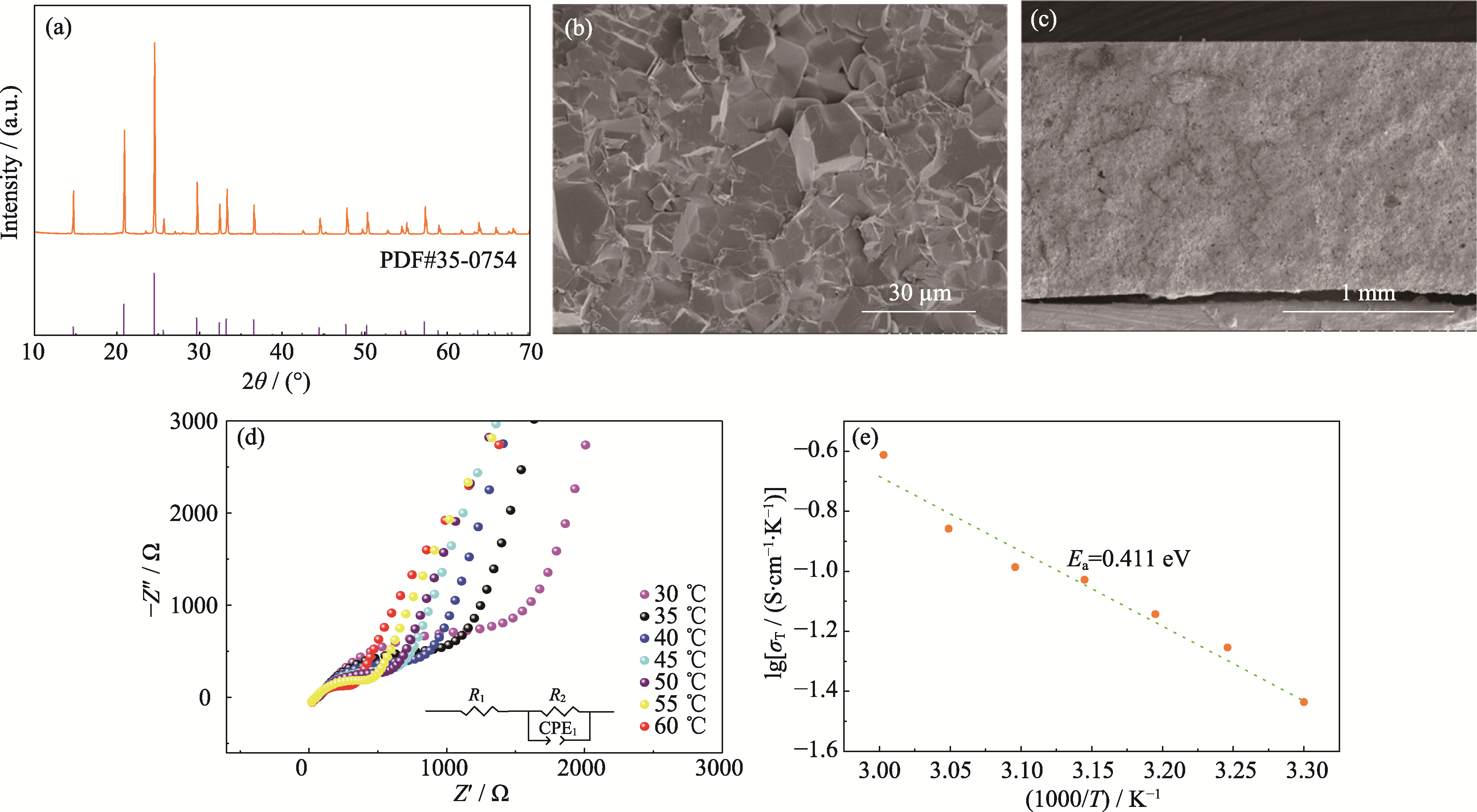
Fig. 1 Basic characteristics of LATP (a) XRD pattern of LATP; (b, c) Cross-sectional SEM images of LATP electrolyte; (d) AC impedance spectra of LATP at different temperatures and corresponding equivalent circuit; (e) Arrhenius plots based on LATP ionic conductivities
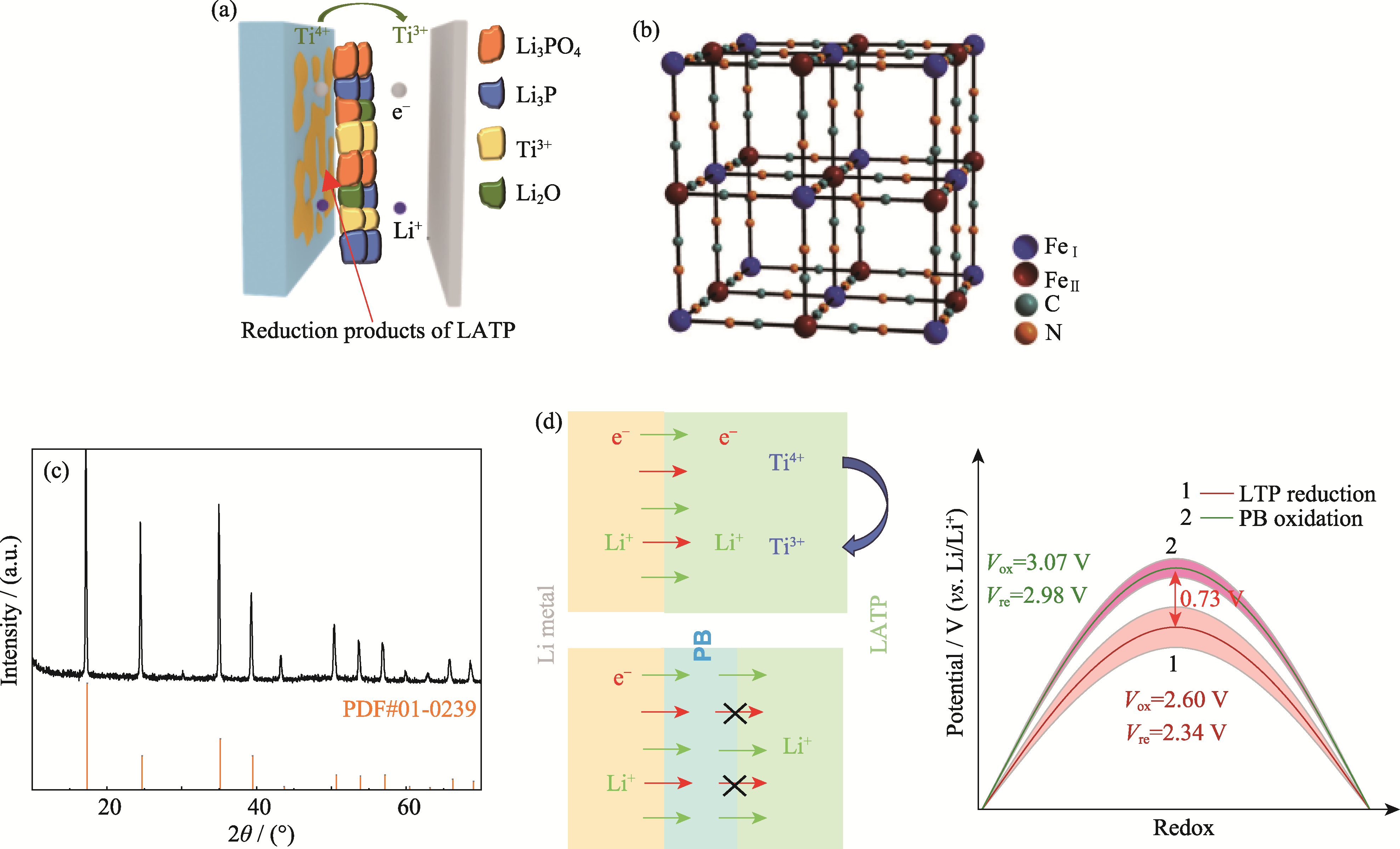
Fig. 2 Basic characteristics of PB and its modification mechanism (a) Schematic diagram of LATP interface reaction and corresponding products; (b) Schematic diagram of PB structure; (c) XRD pattern of PB; (d) Modulation mechanism of PB interface on Li-ion and electron flux
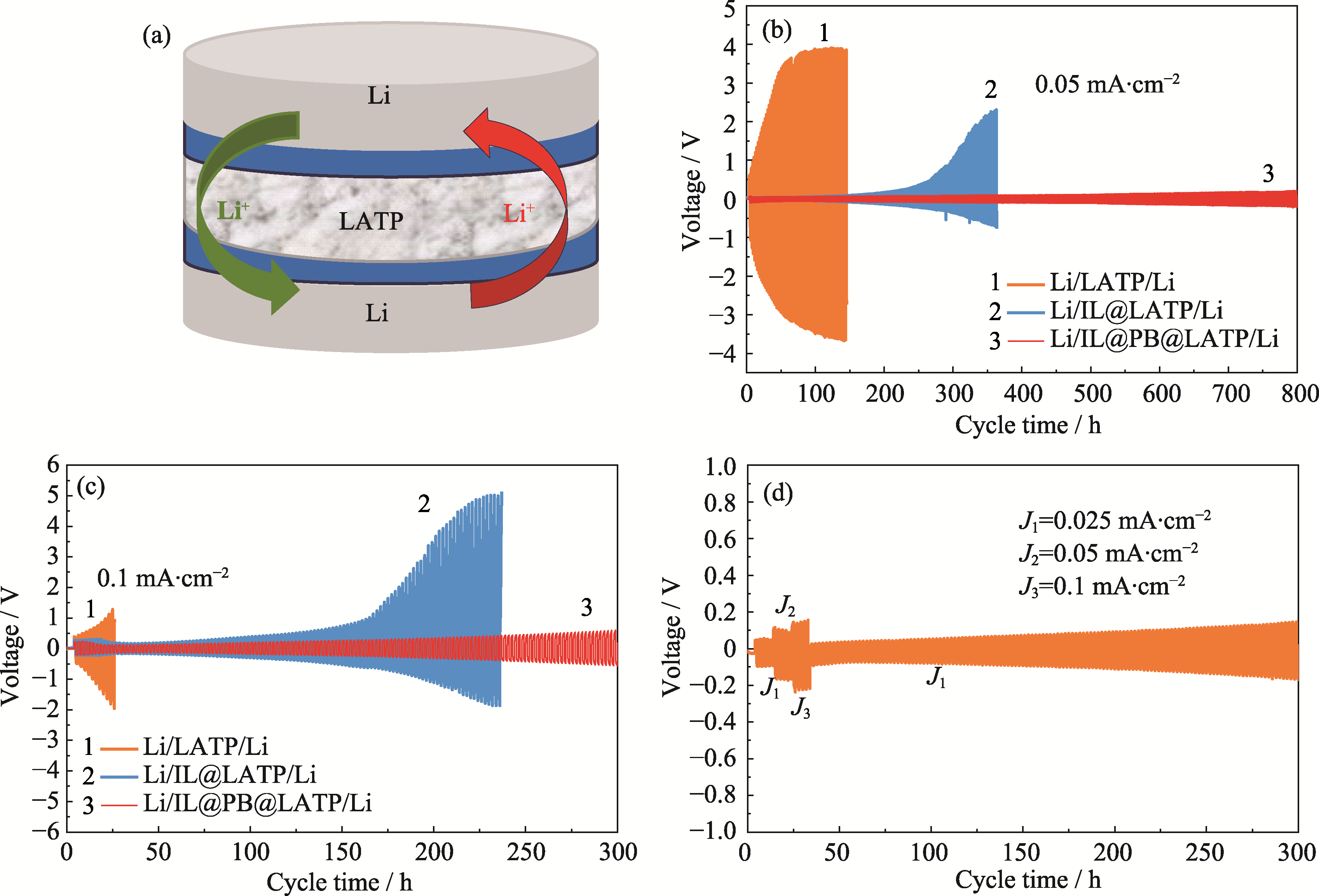
Fig. 3 Schematic and electrochemical performance of symmetric cells (a) Schematic diagram of assembly of Li-Li symmetric cell; (b, c) Li plating and stripping voltage curves of symmetric cells of Li/LATP/Li, Li/IL@LATP/Li, and Li/IL@PB@LATP/Li at current densities of (b) 0.05 and (c) 0.1 mA·cm-2; (d) Li plating and stripping voltage curves of symmetric cell of Li/IL@PB@LATP/Li at different current densities
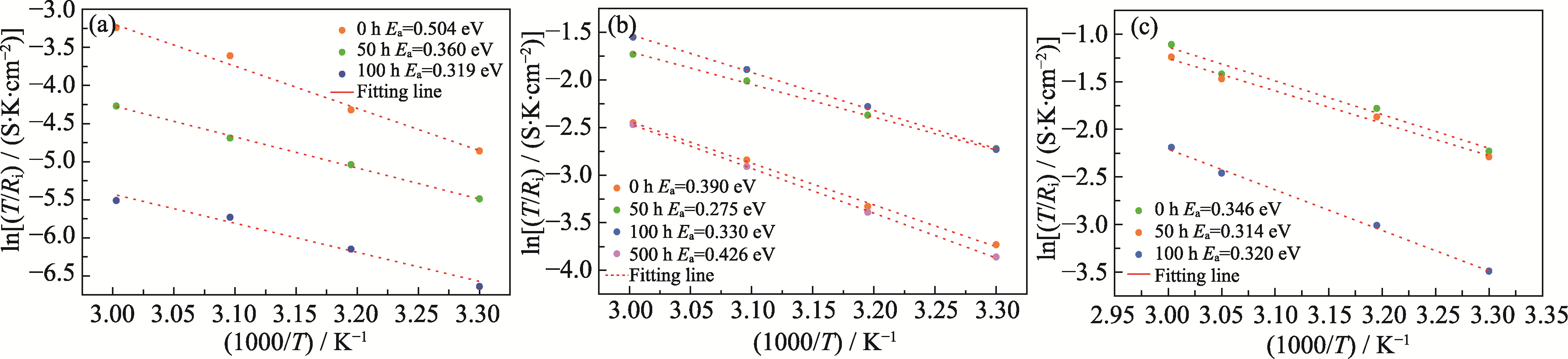
Fig. 4 Arrhenius curves based on interface resistances at different temperatures for symmetric cells of (a) Li/LATP/Li, (b) Li/IL@LATP/Li, and (c) Li/IL@PB@LATP/Li after different cycling time
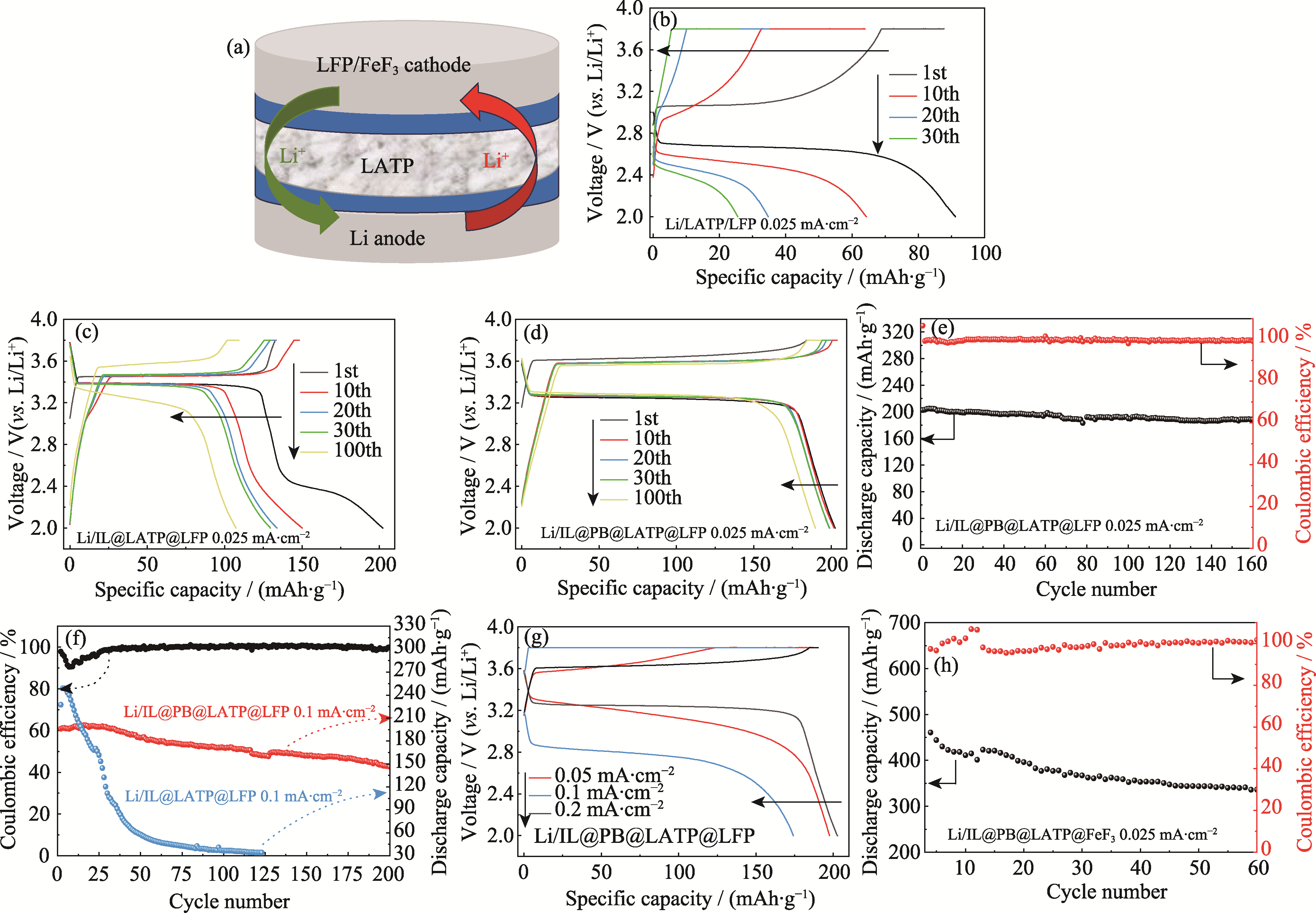
Fig. 5 Schematic diagram of structure and electrochemical performance of Li//LFP and Li//FeF3 full cells under different conditions (a) Schematic diagram of Li//LFP or Li//FeF3 full cell; (b-d) Charging-discharging curves of (b) Li/LATP/LFP, (c) Li/IL@LATP/LFP, and (d) Li/IL@PB@LATP/LFP full cells at different cycle stages under a current density of 0.025 mA·cm-2; (e) Cycling performance of Li/IL@PB@LATP/LFP full cell at a current density of 0.025 mA·cm-2; (f) Cycling performance of Li/IL@LATP/LFP and Li/IL@PB@LATP/LFP full cells at a current density of 0.1 mA·cm-2, and Coulombic efficiency of Li/IL@PB@LATP/LFP; (g) Initial charging-discharging curves of Li/IL@PB@LATP/LFP cell at different current densities; (h) Cycling performance of Li/IL@PB@LATP/FeF3 full cell at 0.025 mA·cm-2
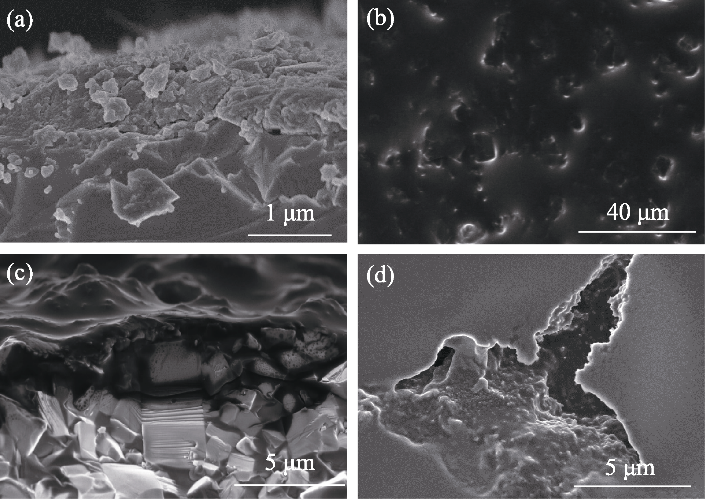
Fig. 6 SEM images of PB@LATP under different conditions (a) Cross-sectional SEM image of PB-modified solid electrolyte LATP; (b) Surface and (c) cross-sectional SEM images of LATP for Li/IL@PB@LATP/LFP full cell after 50 cycles at a current density of 0.05 mA·cm-2; (d) SEM image of LATP surface from Li/IL@LATP/LFP full cell after 50 cycles at a current density of 0.05 mA·cm-2
| [1] |
LIU B, ZHANG J G, XU W. Advancing lithium metal batteries. Joule, 2018, 2(5): 833.
DOI URL |
| [2] |
LI C, CHEN K, ZHOU X, et al. Electrochemically driven conversion reaction in fluoride electrodes for energy storage devices. npj Computational Materials, 2018, 4: 22.
DOI |
| [3] |
YU Y, LAI C, LEI M, et al. Dual strategies of mild C-F scissoring fluorination and local high-concentration electrolyte to enable reversible Li-Fe-F conversion batteries. Materials Horizons, 2024, 11: 2169.
DOI URL |
| [4] |
GU Y, HU J, LEI M, et al. Electrochemical activation of ordered mesoporous solid electrolyte interphases to enable ultra-stable lithium metal batteries. Advanced Energy Materials, 2024, 14(4): 2302174.
DOI URL |
| [5] | WU H, HU J, YU S, et al. Heterostructure conductive interface and melt-penetration-bonding process to afford all-solid-state Li-FeF3 garnet batteries with high cathode loading. Energy & Environmental Science, 2025, 18(2): 923. |
| [6] |
LEE Y, FUJIKI S, JUNG C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nature Energy, 2020, 5: 299.
DOI |
| [7] |
NIE X, LEI M, HU J, et al. Boosting Li-ion conductivity of fluoride solid electrolyte by low-temperature molten salt ablation and particle boundary doping. ACS Nano, 2024, 18(43): 30099.
DOI PMID |
| [8] |
HU J, LEI M, ZHU C, et al. Highly conductive doped fluoride solid electrolytes with solidified ionic liquid to enable reversible FeF3conversion solid state batteries. Advanced Functional Materials, 2024, 34(41): 2314044.
DOI URL |
| [9] |
XIAO Y, WANG Y, BO S, et al. Understanding interface stability in solid-state batteries. Nature Reviews Materials, 2020, 5: 105.
DOI |
| [10] | YU Q, HU J, NIE X, et al. Liquid metal mediated heterostructure fluoride solid electrolytes of high conductivity and air stability for sustainable Na metal batteries. ACS Nano, 2024, 18(7): 5790. |
| [11] |
CHEN R, LI Q, YU X, et al. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chemical Reviews, 2020, 120(14): 6820.
DOI PMID |
| [12] |
YU Q, XU Y, HU J, et al. Heterostructured fluoride-based solid electrolytes engineered by grain boundary softening and bonding for sustainable Na metal batteries. Energy Storage Materials, 2024, 73: 103795.
DOI URL |
| [13] |
LIU Y, LEI M, LAI C, et al. Enable high reversibility of Fe/Cu based fluoride conversion batteries via interfacial gas release and detergency of garnet electrolytes. Materials Today, 2022, 61: 65.
DOI URL |
| [14] |
LIU Y, MENG J, LEI M, et al. Alloyable viscous fluid for interface welding of garnet electrolyte to enable highly reversible fluoride conversion solid state batteries. Advanced Functional Materials, 2023, 33(4): 2208013.
DOI URL |
| [15] |
ZHANG Y, MENG J, CHEN K, et al. Garnet-based solid-state lithium fluoride conversion batteries benefiting from eutectic interlayer of superior wettability. ACS Energy Letters, 2020, 5(4): 1167.
DOI URL |
| [16] | ZHANG Y, MENG J, CHEN K, et al. Behind the candelabra: a facile flame vapor deposition method for interfacial engineering of garnet electrolyte to enable ultralong cycling solid-state Li-FeF3 conversion batteries. ACS Applied Materials & Interfaces, 2020, 12(30): 33729. |
| [17] |
ZENG Y, ZHANG Y, HU J, et al. Garnet-based solid state batteries benefitting from anionic/electronic mixed conductive interface constructed by lithiation of porous FeS2. APL Energy, 2024, 2: 026104.
DOI URL |
| [18] |
LEI M, WU X, LIU Y, et al. Polymer electrolytes reinforced by 2D fluorinated filler for all-solid-state Li-Fe-F conversion-type lithium metal batteries. Nano Research, 2023, 16: 8469.
DOI |
| [19] |
AONO H, SUGIMOTO E, SADAOKA Y, et al. Ionic conductivity of the lithium titanium phosphate (Li1+XMXTi2-X(PO4)3, M=Al, Sc, Y and La) systems. Journal of the Electrochemical Society, 1989, 136(2): 590.
DOI |
| [20] | ZHANG Z, SHAO Y, LOTSCH B V, et al. New horizons for inorganic solid state ion conductors. Energy & Environmental Science, 2018, 11: 1945. |
| [21] |
ZHANG B, TAN R, YANG L, et al. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Materials, 2018, 10: 139.
DOI URL |
| [22] |
YANG Q, Li C. Li metal batteries and solid state batteries benefiting from halogen-based strategies. Energy Storage Materials, 2018, 14: 100.
DOI URL |
| [23] |
HARTMANN P, LEICHTWEISS T, BUSCHE M R, et al. Degradation of NASICON-type materials in contact with lithium metal: formation of mixed conducting interphases (MCI) on solid electrolytes. The Journal of Physical Chemistry C, 2013, 117(41): 21064.
DOI URL |
| [24] |
WANG S, XU H, LI W, et al. Interfacial chemistry in solid-state batteries: formation of interphase and its consequences. Journal of the American Chemical Society, 2018, 140(1): 250.
DOI PMID |
| [25] |
ZHENG F, SONG Z, LI H, et al. Distinct functional Janus interfaces for dendrite-free Li1.3Al0.3Ti1.7(PO4)3-based lithium metal batteries. Electrochimica Acta, 2022, 436: 141395.
DOI URL |
| [26] |
HUANG C, LI Z, DUAN S, et al. Improving the stability of NASICON-type electrolyte with Li metal anode by interfacial modification. Journal of Power Sources, 2022, 536: 231491.
DOI URL |
| [27] |
GU Y, HU J, LAI C, et al. NASICON-based solid state Li-fluoride conversion batteries enabled by constructing a fluorine-rich trap for Ti4+. Advanced Energy Materials, 2023, 13(12): 2203679.
DOI URL |
| [28] |
LEI M, FAN S, YU Y, et al. NASICON-based solid state Li-Fe-F conversion batteries enabled by multi-interface-compatible sericin protein buffer layer. Energy Storage Materials, 2022, 47: 551.
DOI URL |
| [29] |
WU C, HU J, YANG Q, et al. Open framework perovskite derivate SEI with fluorinated heterogeneous nanodomains for practical Li-metal pouch cells. Nano Energy, 2023, 113: 108523.
DOI URL |
| [30] |
PAOLELLA A, FAURE C, TIMOSHEVSKII V, et al. A review on hexacyanoferrate-based materials for energy storage and smart windows: challenges and perspectives. Journal of Materials Chemistry A, 2017, 5: 18919.
DOI URL |
| [31] |
ZHANG B, ZHANG Y, LEI M, et al. Lithiation-induced conductivity modulation in Prussian blue interlayer for stable Li/garnet solid-state batteries. Applied Physics Letters, 2023, 122(3): 033901.
DOI URL |
| [32] |
CHEN K, QIU W, LEI M, et al. Manipulating cation-anion coordination in fire-retardant electrolytes to enable high-areal- capacity fluoride conversion batteries. Matter, 2024, 7(11): 3907.
DOI URL |
| [33] | CHEN K, LEI M, YAO Z, et al. Construction of solid-liquid fluorine transport channel to enable highly reversible conversion cathodes. Science Advances, 2021, 7(45): abj1491. |
| [34] |
WU X, ZHENG Y, LI W, et al. Solid electrolytes reinforced by infinite coordination polymer nano-network for dendrite-free lithium metal batteries. Energy Storage Materials, 2023, 41: 436.
DOI URL |
| [35] |
HU J, LAI C, CHEN K, et al. Dual fluorination of polymer electrolyte and conversion-type cathode for high-capacity all-solid- state lithium metal batteries. Nature Communications, 2022, 13: 7914.
DOI |
| [1] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [2] | WANG Kunpeng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on Water-minimal Prussian Blue Cathode [J]. Journal of Inorganic Materials, 2024, 39(9): 1005-1012. |
| [3] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [4] | TAN Shuyu, LIU Xiaoning, BI Zhijie, WAN Yong, GUO Xiangxin. Jointing of Cathode Coating and Interface Modification for Stabilizing Poly(ethylene oxide) Electrolytes Against High-voltage Cathodes [J]. Journal of Inorganic Materials, 2023, 38(12): 1466-1474. |
| [5] | DU Qiujing, LIU Tianzhi, CHEN Jufeng, CHEN Hangrong. Construction of Prussian Blue Fluorescent Nanoprobe for Specific Detection of HClO in Cancer Cells [J]. Journal of Inorganic Materials, 2023, 38(1): 55-61. |
| [6] | ZHANG Jiaqiang, ZOU Xinlei, WANG Nengze, JIA Chunyang. Zn-Fe PBA Films by Two-step Electrodeposition Method: Preparation and Performance in Electrochromic Devices [J]. Journal of Inorganic Materials, 2022, 37(9): 961-968. |
| [7] | XIA Qiuying, SUN Shuo, ZAN Feng, XU Jing, XIA Hui. Amorphous LiSiON Thin Film Electrolyte for All-solid-state Thin Film Lithium Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 230-236. |
| [8] | LI Dong, LEI Chao, LAI Hua, LIU Xiao-Lin, YAO Wen-Li, LIANG Tong-Xiang, ZHONG Sheng-Wen. Recent Advancements in Interface between Cathode and Garnet Solid Electrolyte for All Solid State Li-ion Batteries [J]. Journal of Inorganic Materials, 2019, 34(7): 694-702. |
| [9] | Yong LI, Wei-Xin HE, Xin-Yue ZHENG, Sheng-Lan YU, Hai-Tong LI, Hong-Yi LI, Rong ZHANG, Yu WANG. Prussian Blue Cathode Materials for Aqueous Sodium-ion Batteries:Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2019, 34(4): 365-372. |
| [10] | LU Dong-Liang, DAI Guang-Zhou, YAO Ying-Bang, TAO Tao, LIANG Bo, LU Sheng-Guo. Influence of Calcining Temperature on the Property of Li0.33La0.56TiO3 Solid-state Ionic Capacitor [J]. Journal of Inorganic Materials, 2018, 33(10): 1077-1082. |
| [11] | XU Shun-Jian, LUO Yu-Feng, ZHONG Wei, XIAO Zong-Hu, LOU Yong-Ping, OU Hui. Quasi-solid-state Dye-sensitized Solar Cells Employing Fibers Stacked Paper Carbons as Efficient Counter Electrodes [J]. Journal of Inorganic Materials, 2015, 30(1): 29-34. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||