
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (10): 1200-1206.DOI: 10.15541/jim20230072
Special Issue: 【结构材料】核用陶瓷(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
GU Junyi1,2( ), FAN Wugang1, ZHANG Zhaoquan1(
), FAN Wugang1, ZHANG Zhaoquan1( ), YAO Qin1(
), YAO Qin1( ), ZHAN Hongquan2
), ZHAN Hongquan2
Received:2022-02-13
Revised:2023-03-29
Published:2023-10-20
Online:2023-06-01
Contact:
ZHANG Zhaoquan, professor. E-mail: zhangzq@mail.sic.ac.cn;About author:GU Junyi (1997-), male, Master candidate. E-mail: 2020028013@stu.jci.edu.cn
Supported by:CLC Number:
GU Junyi, FAN Wugang, ZHANG Zhaoquan, YAO Qin, ZHAN Hongquan. Preparation and Thermal Property of PrAlO3 Ceramics[J]. Journal of Inorganic Materials, 2023, 38(10): 1200-1206.
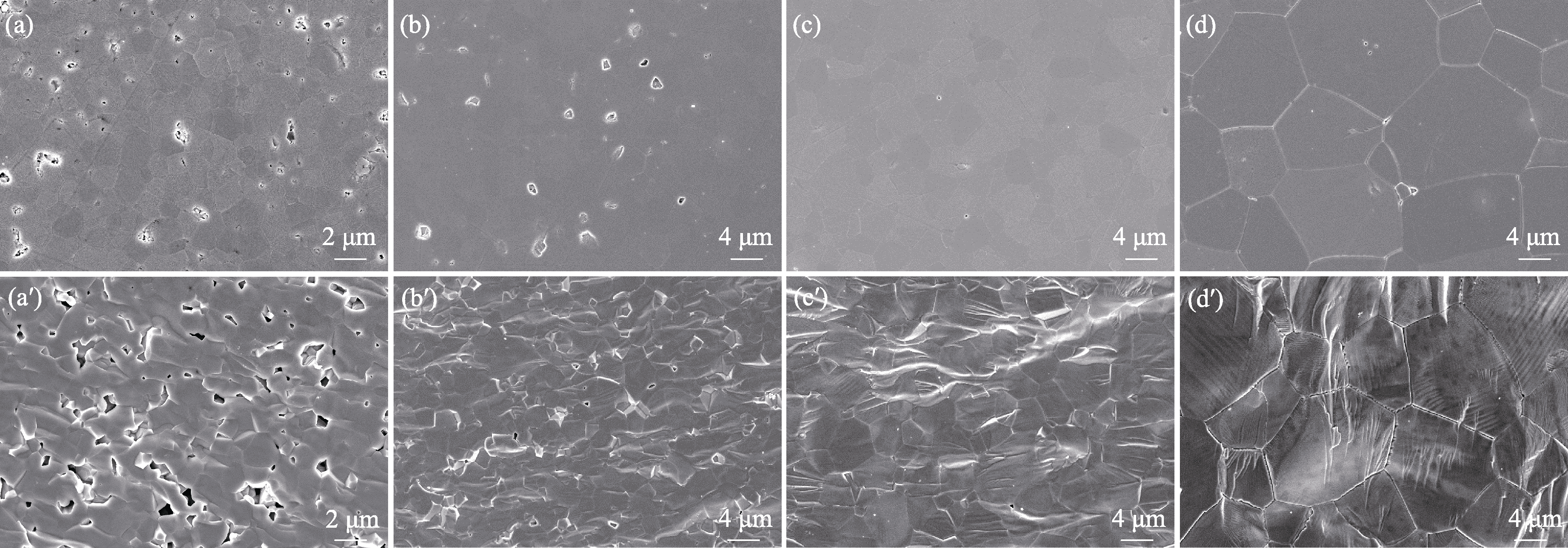
Fig. 5 SEM images of (a-d) areal surface and (a′-d′) fractural surface of T4 samples sintered at different temperatures (a, a′) 1430 ℃; (b, b′) 1460 ℃; (c, c′) 1500 ℃; (d, d′) 1600 ℃
| Sintering temperature/℃ | Bending strength/MPa | Fracture toughness/(MPa·m1/2) | Vickers hardness/GPa | Average grain size/μm |
|---|---|---|---|---|
| 1500 | 88.47±4.14 | 0.81±0.02 | 7.95±0.43 | 5.51±1.84 |
| 1600 | 95.55±4.62 | 0.92±0.06 | 7.75±0.28 | 15.40±5.02 |
Table 1 Mechanical property of PrAlO3 prepared at different sintering temperatures
| Sintering temperature/℃ | Bending strength/MPa | Fracture toughness/(MPa·m1/2) | Vickers hardness/GPa | Average grain size/μm |
|---|---|---|---|---|
| 1500 | 88.47±4.14 | 0.81±0.02 | 7.95±0.43 | 5.51±1.84 |
| 1600 | 95.55±4.62 | 0.92±0.06 | 7.75±0.28 | 15.40±5.02 |

Fig. 7 Thermophysical property of sample T4 sintered at different temperatures (a) Thermal expansion coefficient; (b) Thermal diffusivity; (c) Specific heat capacity; (d) Thermal conductivity
| Material | Density/ (g·cm-3) | Thermal expansion coefficient/ (×10-6, K-1) | Thermal conductivity/ (W·m-1·K-1) |
|---|---|---|---|
| PrAlO3 | 6.69 | 9.3 | 5.0 |
| GdAlO3[ | 6.64 | 4.8±0.2 | 3.5±0.1 |
| Tb2TiO5[ | 6.89 | 6.8±0.2 | 1.3±0.1 |
| B4C[ | 2.25 | 3.7±0.1 | 9.8±0.1 |
| Ag-In-Cd[ | 10.18 | 22.5 (25~500 ℃) | 92.0±1.0 |
Table 2 Thermal expansion coefficient and thermal conductivity parameters of different control rods at 360 ℃
| Material | Density/ (g·cm-3) | Thermal expansion coefficient/ (×10-6, K-1) | Thermal conductivity/ (W·m-1·K-1) |
|---|---|---|---|
| PrAlO3 | 6.69 | 9.3 | 5.0 |
| GdAlO3[ | 6.64 | 4.8±0.2 | 3.5±0.1 |
| Tb2TiO5[ | 6.89 | 6.8±0.2 | 1.3±0.1 |
| B4C[ | 2.25 | 3.7±0.1 | 9.8±0.1 |
| Ag-In-Cd[ | 10.18 | 22.5 (25~500 ℃) | 92.0±1.0 |
| [1] |
HWANG J, RAO R R, GIORDANO L, et al. Perovskites in catalysis and electrocatalysis. Science, 2017, 358(6364): 751.
DOI PMID |
| [2] |
BOZTOSUN I, ĐAPO H, KARAKOÇ M. Measuring decay of praseodymium isotopes activated by a clinical LINAC. Modern Physics Letters A, 2016, 31(36): 1650212.
DOI URL |
| [3] |
WADUGE W L I, CHEN Y, ZUO P, et al. Solid-phase epitaxy of perovskite high dielectric PrAlO3 films grown by atomic layer deposition for use in two-dimensional electronics and memory devices. ACS Applied Nano Materials, 2019, 2(11): 7449.
DOI URL |
| [4] | EMERY A A, WOLVERTON C. High-throughput DFT calculations of formation energy, stability and oxygen vacancy formation energy of ABO3 perovskites. Scientific Data, 2017, 4: 170153. |
| [5] |
COHEN E, RISBERG L A, NORDLAND W A, et al. Structural phase transitions in PrAlO3. Physical Review, 1969, 186(2): 476.
DOI URL |
| [6] |
HARLEY R T, HAYES W, PERRY A M, et al. The phase transitions of PrAlO3. Journal of Physics C: Solid State Physics, 1973, 6(14): 2382.
DOI URL |
| [7] |
HARLEY R T. Mechanism of 118.5 K phase transition in PrAlO3. Journal of Physics C: Solid State Physics, 1977, 10(8): L205.
DOI URL |
| [8] |
HOWARD C J, KENNEDY B J, CHAKOUMAKOS B C. Neutron powder diffraction study of rhombohedral rare-earth aluminates and the rhombohedral to cubic phase transition. Journal of Physics- Condensed Matter, 2000, 12(4): 349.
DOI URL |
| [9] | MOUSSA S M, KENNEDY B J, HUNTER B A, et al. Low temperature structural studies on PrAlO3. Journal of Physics- Condensed Matter, 2001, 13(9): L203. |
| [10] |
PAWLAK D A, LUKASIEWICZ T, CARPENTER M, et al. Czochralski crystal growth, microstructure and spectroscopic properties of PrAlO3 perovskite. Journal of Crystal Growth, 2005, 282(1/2): 260.
DOI URL |
| [11] |
NOVOSELOV A, YOSHIKAWA A, SOLOVIEVA N, et al. Crystal growth, optical and luminescence properties of (Ce,Sr)-doped PrAlO3 single crystals. Crystal Research and Technology, 2007, 42(12): 1320.
DOI URL |
| [12] |
WENCKA M, VRTNIK S, JAGODIC M, et al. Observation of anomalous magnetism in the low-temperature monoclinic phase of single-crystalline PrAlO3 perovskite. Physical Review B, 2009, 80(22): 224410.
DOI URL |
| [13] |
SHRIVASTAVA V, NAGARAJAN R. Consequences of Bi3+ introduction for Pr3+ in PrAlO3. Journal of Materials Science, 2020, 55(32): 15415.
DOI |
| [14] |
KIM C H, CHO S Y, KIM I T, et al. Twin structures in lanthanum, praseodymium, and neodymium aluminate ceramics. Materials Research Bulletin, 2001, 36(9): 1561.
DOI URL |
| [15] | GAZULLA M F, VENTURA M J, ANDREU C, et al. Praseodymium oxides. complete characterization by determining oxygen content. Microchemical Journal, 2019, 148: 291. |
| [16] |
FU S, YANG Z C, LI H H, et al. Effects of Gd2O3 and MgSiN2 sintering additives on the thermal conductivity and mechanical properties of Si3N4 ceramics. International Journal of Applied Ceramic Technology, 2023, 20(3): 1855.
DOI URL |
| [17] |
CHEN C, YI X Z, ZHANG S, et al. Vacuum sintering of Tb3Al5O12 transparent ceramics with combined TEOS+MgO sintering aids. Ceramics International, 2015, 41(10): 12823.
DOI URL |
| [18] |
JIA W T, WEI Q L, ZHANG H B, et al. Comparative analyses of the influence of tetraethoxysilane additives on the sintering kinetics of Nd:YAG transparent ceramics. Journal of Materials Science-Materials in Electronics, 2021, 32(14): 19218.
DOI |
| [19] |
TONG H Z, WANG N L, ZOU Y Q, et al. Densification and mechanical properties of YAG ceramics fabricated by air pressureless sintering. Journal of Electronic Materials, 2019, 48(1): 374.
DOI |
| [20] |
KINSMAN K M, MCKITTRICK J. Phase development and luminescence in chromium-doped yttrium aluminum garnet (YAG:Cr) phosphors. Journal of the American Ceramic Society, 1994, 77(11): 2866.
DOI URL |
| [21] |
KLEMM H. Silicon nitride for high-temperature applications. Journal of the American Ceramic Society, 2010, 93(6): 1501.
DOI URL |
| [22] | ZHANG Y N. Co-precipitation Synthesis of Yb:YAG Nano-powders and Fabrication of Yb:YAG Transparent Ceramics. Shenyang: Master's Thesis of Northeastern University, 2015. |
| [23] |
STEVENSON A J, LI X, MARTINEZ M A, et al. Effect of SiO2 on densification and microstructure development in Nd:YAG transparent ceramics. Journal of the American Ceramic Society, 2011, 94(5): 1380.
DOI URL |
| [24] |
KOLITSCH U, SEIFERT H J, LUDWIG T, et al. Phase equilibria and crystal chemistry in the Y2O3-Al2O3-SiO2 system. Journal of Materials Research, 1999, 14(2): 447.
DOI URL |
| [25] | LU G Z, SUN X D. Phase transformation, sintering and microstructures of transparent YAG ceramics doping TEOS. Chinese Journal of Nonferrous Metals, 2010, 20(6): 1220. |
| [26] | XU G. Sintering Additives in Transparent Nd:YAG Ceramics in the Role and Performance Characterization. Xi'an: Master's Thesis of Xidian University, 2014. |
| [27] |
KUZJUKĒVIČS A, ISHIZAKI K. Sintering of silicon nitride with YAIO3 additive. Journal of the American Ceramic Society, 1993, 76(9): 2373.
DOI URL |
| [28] |
YANG Q Y, QIU P F, SHI X, et al. Application of entropy engineering in thermoelectrics. Journal of Inorganic Materials, 2021, 36(4): 347.
DOI |
| [29] |
GUO X, FENG Y R, ZHAO J X, et al. Neutron adsorption performance of Dy2TiO5 materials obtained from powders synthesized by the molten salt method. Ceramics International, 2017, 43(2): 1975.
DOI URL |
| [30] | AGENCY I. Absorber materials, control rods and designs of shutdown systems for advanced liquid metal fast reactors. Proceeding of a Technical Committee Meeting, 1996: 169. |
| [31] |
KIM H S, JOUNG C Y, LEE B H, et al. Characteristics of GdxMyOz (M=Ti, Zr or Al) as a burnable absorber. Journal of Nuclear Materials, 2008, 372(2/3): 340.
DOI URL |
| [32] |
HUANG J H, RAN G, LIU T J, et al. Microstructure and physical properties of Tb2TiO5 neutron absorber synthesized by ball milling and sintering. Journal of Materials Engineering and Performance, 2016, 25(10): 4266.
DOI URL |
| [33] | SU X P, DU A B, HAN X H, et al. Study on thermophysical properties of B4C pellets used as control material in fast reactor. Shandong Ceramics, 2015, 38(4): 3. |
| [34] | RUDY J M K. Comprehensive Nuclear Materials. Amsterdam: Elsevier, 2020, 7: 533. |
| [35] | XIAO H X, LONG C S. Effect of Sn Addition to Ag-In-Cd Alloy on the Microstructure and Thermo-physical Properties. 2013 Annual Conference of Chinese Nuclear Society, Harbin, 2013: 302-305. |
| [36] |
GU J Y, FAN W G, ZHANG Z Q, et al. Structure and optical property of Pr2O3 powder prepared by reduction. Journal of Inorganic Materials, 2023, 38(7): 771.
DOI URL |
| [37] |
ZHANG Q Y, HUANG X Y. Recent progress in quantum cutting phosphors. Progress in Materials Science, 2010, 55(5): 353.
DOI URL |
| [1] | CAI An,XIE Hua-Qing,XI Tong-Geng. Thermophysical Properties of Eleven Thermal Control Materials Related to Processing Conditions and Microstructure for Spacecraft [J]. Journal of Inorganic Materials, 2006, 21(5): 1173-1178. |
| [2] | ZHOU Zong-Hui,DU Pi-Yi,WENG Wen-Jian,HAN Gao-Rong,SHEN Ge. Formation and Properties of the BSTN Composite Ceramics with Perovskite and Tungsten Bronze Phases [J]. Journal of Inorganic Materials, 2004, 19(6): 1322-1328. |
| [3] | CUI Bin,TIAN Chang-Sheng,SHI Qi-Zhen. 0.80Pb(Mg1/3Nb2/3)O3-0.20PbTiO3 Ceramics Prepared by Semichemical Method [J]. Journal of Inorganic Materials, 2004, 19(6): 1313-1321. |
| [4] | CUI Bin,YANG Zu-Pei,HOU Yu-Dong,SHI Qi-Zhen,TIAN Chang-Sheng. Reaction Mechanism of 0.80Pb(Mg1/3Nb2/3)O3 -0.20PbTiO3 Ceramics Prepared by Semichemical Method [J]. Journal of Inorganic Materials, 2002, 17(4): 737-744. |
| [5] | XI Tonggeng,WANG Shengmei,ZHANG Zhongde,LU Yanjing,LI Minghua. A Prediction and Optimization of Thermophysical Properties for High temperature Thermal Insulation Materials [J]. Journal of Inorganic Materials, 1997, 12(2): 207-210. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||