
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (4): 429-436.DOI: 10.15541/jim20220594
Special Issue: 【信息功能】忆阻器材料与器件(202506)
• Topical Section on Neuromorphic Materials and Devices (Contributing Editor: WAN Qing) • Previous Articles Next Articles
LI Yanran( ), XIE Dingdong, JIANG Jie(
), XIE Dingdong, JIANG Jie( )
)
Received:2022-10-11
Revised:2022-11-08
Published:2023-04-20
Online:2023-04-18
Contact:
JIANG Jie, associate professor. E-mail: jiangjie@csu.edu.cnAbout author:LI Yanran (1996-), female, PhD candidate. E-mail: 222201001@csu.edu.cn
Supported by:CLC Number:
LI Yanran, XIE Dingdong, JIANG Jie. Bionic Research on Multistage Pain Sensitization Based on Ionic Oxide Transistor Array[J]. Journal of Inorganic Materials, 2023, 38(4): 429-436.
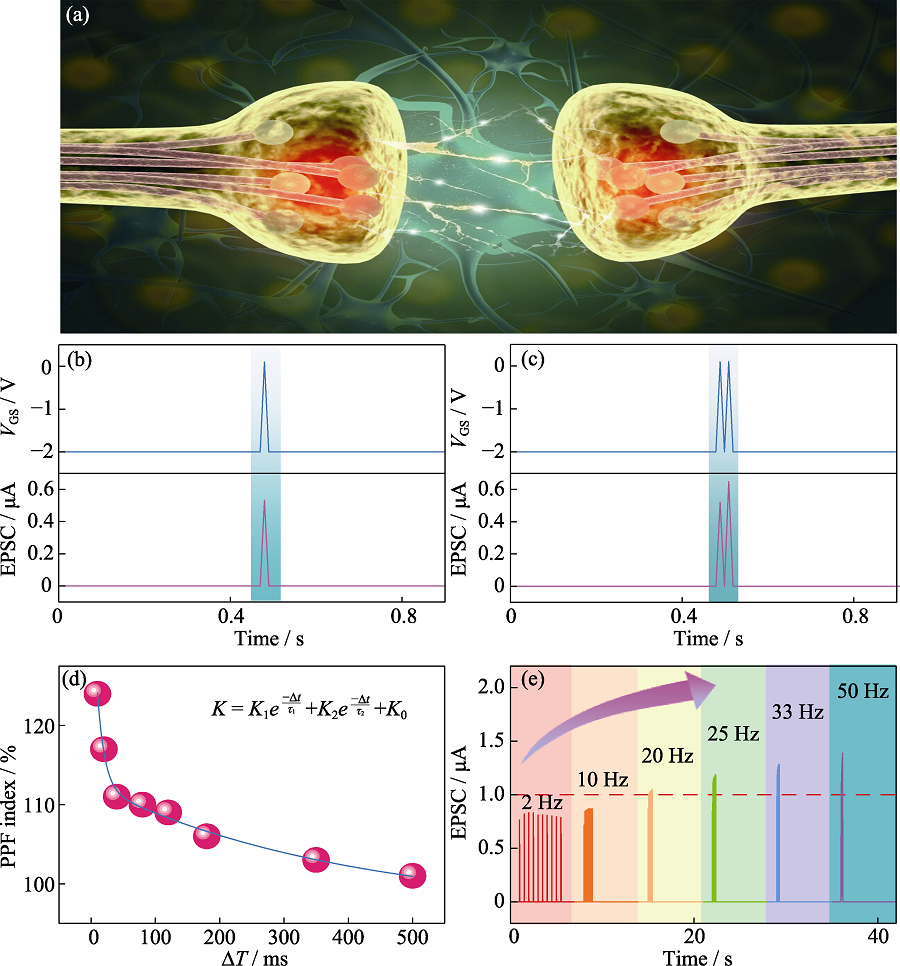
Fig. 2 (a) Schematic image of a biological synapse, (b) EPSC and (c) PPF triggered by a presynaptic spike with amplitude at 2.10 V, duration at 10 ms, (d) PPF index fitting, and (e) EPSCs recorded in response to the different stimulus train with frequency ranging from 2 Hz to 50 Hz
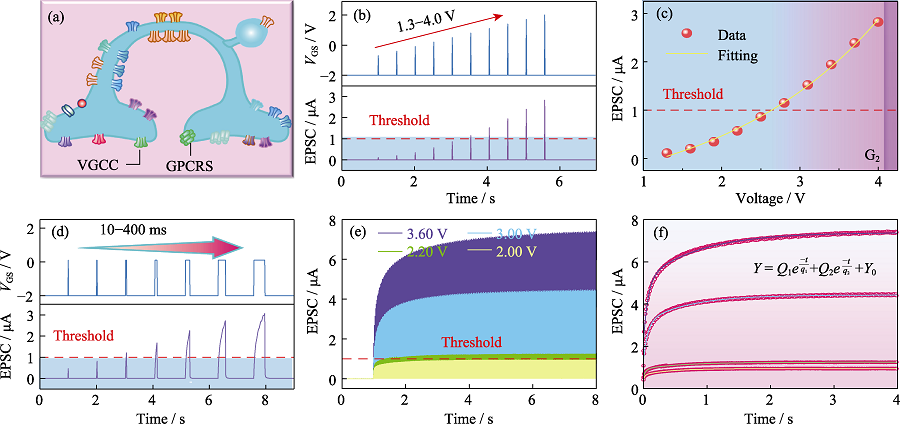
Fig. 3 Characteristics of the artificial painful perceptual neuron (a) Structural diagram of painful perceptual neuron; (b) EPSC response by the device applying ten electrical pulses with 10 ms pulse width and different pulse amplitudes from 1.30 V to 4.00 V,which cannot reach threshold current (Ith = 1 µA) until the pulse amplitude up to 2.64 V; (c) Fitting curve of pain threshold voltage; (d) EPSC output by the device with fixed pulse amplitude (2.10 V) and the width increasing from 10 ms to 400 ms; (e) Response of device to continuous multiple pulses with different amplitudes; (f) Fitting for response of device to continuous multiple pulses with different amplitudes (2.00, 2.20, 3.00 and 3.60 V); Colorful figures are available on website
| Voltage/V | Y0 /μA | Q1/μA | Q2/μA | q1/s | q2/s |
|---|---|---|---|---|---|
| 3.60 | 7.38 | -2.89 | -2.26 | 0.07 | 0.86 |
| 3.00 | 4.43 | -1.79 | -1.31 | 0.06 | 0.68 |
| 2.20 | 1.25 | -0.35 | -0.31 | 0.04 | 0.62 |
| 2.00 | 0.95 | -0.22 | -0.29 | 0.03 | 0.42 |
Table 1 Fitting results of different parameters in Fig. 3(f)
| Voltage/V | Y0 /μA | Q1/μA | Q2/μA | q1/s | q2/s |
|---|---|---|---|---|---|
| 3.60 | 7.38 | -2.89 | -2.26 | 0.07 | 0.86 |
| 3.00 | 4.43 | -1.79 | -1.31 | 0.06 | 0.68 |
| 2.20 | 1.25 | -0.35 | -0.31 | 0.04 | 0.62 |
| 2.00 | 0.95 | -0.22 | -0.29 | 0.03 | 0.42 |
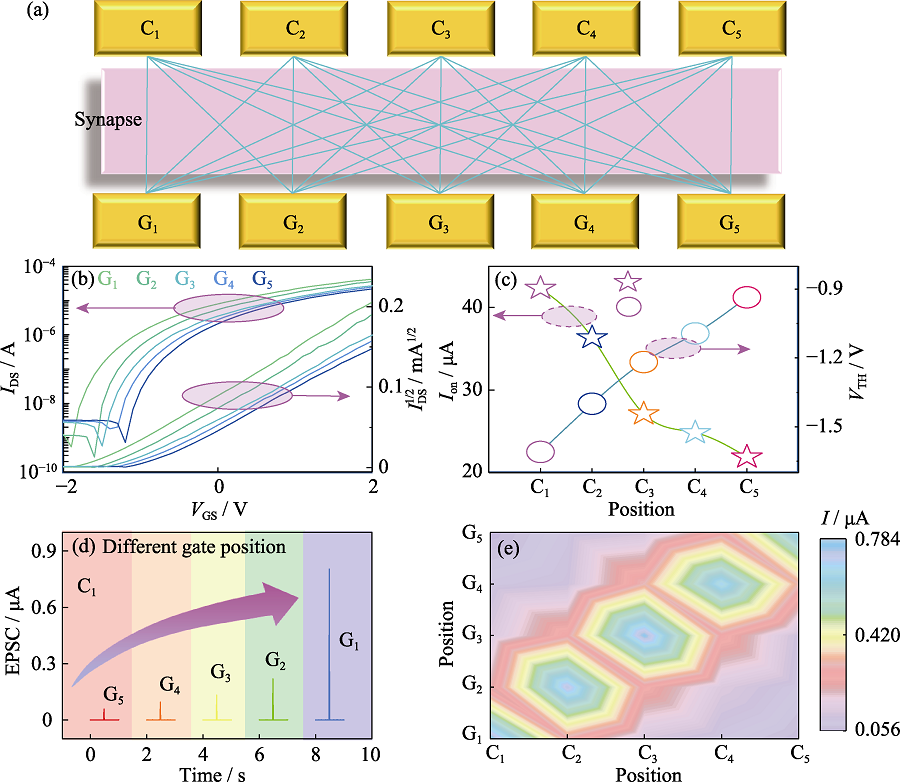
Fig. 4 Nooceptor network and its pain perception and sensitization function (a) Schematic diagram of the junctionless transistor array used to construct the nociceptor network; (b) Transfer curves of channel C1 corresponding to 5 different grate positions; (c) Statistical results of Ion and VTH corresponding to the different gates; (d) EPSC response at different grate positions corresponding to C1 under fixed grate voltage (VGS=1.80 V); (e) EPSC response of five channels (C1-C5) corresponding to five different grid positions (G1-G5); Colorful figures are available on website
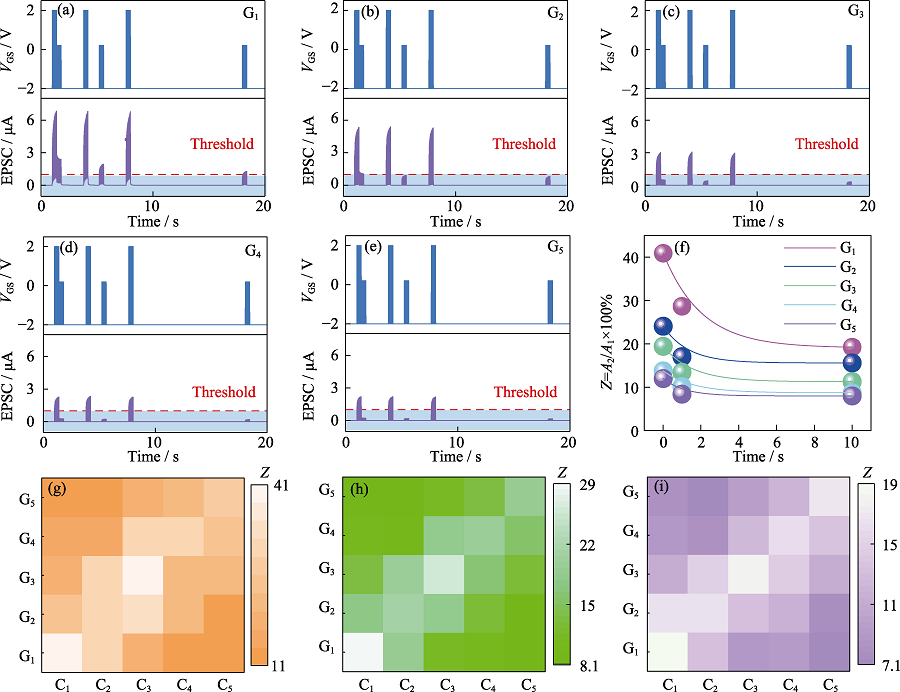
Fig. 5 Response of EPSC with two pulse sequence (4.00 and 2.20 V) applied in (a) G1, (b) G2, (c) G3, (d) G4, and (e) G5; (f) Relationship between sensitization Z and gate position and pulse sequence interval; (g-i) Relationship between sensitization Z(B2/B1) and gate position in the array at different time intervals ((g) 0.01s, (h) 1.00 s, (i) 10.00 s)
| Position | Z0/% | N0/% | τ/s |
|---|---|---|---|
| G1 | 19.08 | 21.90 | 2.02 |
| G2 | 15.60 | 8.53 | 1.32 |
| G3 | 11.28 | 8.20 | 1.55 |
| G4 | 8.72 | 5.08 | 1.60 |
| G5 | 8.00 | 4.03 | 1.13 |
Table 2 Fitting results of different parameters in Fig. 5(f)
| Position | Z0/% | N0/% | τ/s |
|---|---|---|---|
| G1 | 19.08 | 21.90 | 2.02 |
| G2 | 15.60 | 8.53 | 1.32 |
| G3 | 11.28 | 8.20 | 1.55 |
| G4 | 8.72 | 5.08 | 1.60 |
| G5 | 8.00 | 4.03 | 1.13 |
| [1] |
BASBAUM A I, BAUTISTA D M, SCHERRER G, et al. Cellular and molecular mechanisms of pain. Cell, 2009, 139(2):267.
DOI PMID |
| [2] | CORTELLI P, GIANNINI G, FAVONI V, et al. Nociception and autonomic nervous system. Neurological Sciences, 2013, 34(1):41. |
| [3] |
RAJA S N, CARR D B, COHEN M, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain, 2020, 161(9): 1976.
DOI URL |
| [4] |
TRACEY J R, DANIEL W. Nociception. Current Biology, 2017, 27(4):129.
DOI PMID |
| [5] |
LI Y, YIN K, JIANG J, et al. A biopolymer-gated ionotronicjunctionless oxide transistor array for spatiotemporal pain-perception emulation in nociceptor network. Nanoscale, 2022, 14(6):2316.
DOI URL |
| [6] |
LI F, GAO S, LU Y, et al. Bio-inspired multi-mode pain-perceptual system (MMPPS) with noxious stimuli warning, damage localization, and enhanced damage protection. Advanced Science, 2021, 8(10):2004208.
DOI URL |
| [7] | LU Q, SUN F, LIU L, et al. Bio-inspired flexible artificial synapses for pain perception and nerve injuries. npj Flexible Electronics, 2020, 4: 3. |
| [8] | KUMAR M, KIM H S, KIM J. A highly transparent artificial photonic nociceptor. Advanced Materials, 2019, 31(19):e1900021. |
| [9] |
GE J, ZHANG S, LIU Z, et al. Flexible artificial nociceptor using a biopolymer-based forming-free memristor. Nanoscale, 2019, 11(14):6591.
DOI PMID |
| [10] |
WANG C, LIANG S, WANG S, et al. Gate-tunable van der waals heterostructure for reconfigurable neural network vision sensor. Science Advances, 2020, 6(26):eaba6173.
DOI URL |
| [11] |
CHEN Y, SHU Z, ZHANG S, et al. Sub-10 nm fabrication: methods and applications. International Journal of Extreme Manufacturing, 2021, 3(3):032002.
DOI |
| [12] |
XIE D, YIN K, JANG J, et al. Polarization-perceptual anisotropic two-dimensional ReS2 neuro-transistor with reconfigurable neuromorphic vision. Materials Horizons, 2022, 9(5):1448.
DOI URL |
| [13] |
YUKIHIRO K, YU N, MICHIHITO U. Ferroelectric artificial synapses for recognition of a multishaded image. IEEE Transactions on Electron Devices, 2014, 61(8):2827.
DOI URL |
| [14] |
JIANG J, GUO J, WAN X, et al. 2D MoS2 neuromorphic devices for brain-like computational systems. Small, 2017, 13(29):1700933.
DOI URL |
| [15] |
JANG J, HU W, XIE D, et al. 2D electric-double-layer phototransistor for photoelectronic and spatiotemporal hybrid neuromorphic integration. Nanoscale, 2019, 11(3):1360.
DOI PMID |
| [16] |
LUO C, KUNER T, KUNER R. Synaptic plasticity in pathological pain. Trends in Neurosciences, 2014, 37(6):343.
DOI PMID |
| [17] |
CHANG Y, SHAN K, XU Y, et al. Hardware implementation of photoelectrically modulated dendritic arithmetic and spike-timing-dependent plasticity enabled by an ion-coupling gate-tunable vertical 0D-perovskite/2D-MoS2 hybrid-dimensional van der Waals heterostructure. Nanoscale, 2020, 12(42):21798.
DOI URL |
| [18] |
WANG Y Q, WANG J, XIA S H, et al. Neuropathic pain generates silent synapses in thalamic projection to anterior cingulate cortex. Pain, 2021, 162(5):1322.
DOI PMID |
| [19] |
HU W, JIANG J, XIE D, et al. Proton-electron-coupled MoS2 synaptic transistors with a natural renewable biopolymer neurotransmitter for brain-inspired neuromorphic learning. Journal of Materials Chemistry C, 2019, 7(3):682.
DOI URL |
| [20] | XIE D, JING J, HU W, et al. Coplanar multigate MoS2 electric-double-layer transistors for neuromorphic visual recognition. ACS Appled Materials Interfaces, 2018, 10(31):25943. |
| [21] |
CITRI A, MALENKA R C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology, 2008, 33(1):18.
DOI PMID |
| [22] |
FENG G, JIANG J, ZHAO Y, et al. A sub-10 nm vertical organic/ inorganic hybrid transistor for pain-perceptual and sensitization-regulated nociceptor emulation. Advanced Materials, 2020, 32(6):1906171.
DOI URL |
| [23] |
GOLD M S, GEBHART G F. Nociceptor sensitization in pain pathogenesis. Nature Medicine, 2010, 16(11):1248.
DOI PMID |
| [24] |
JIANG S, HE Y, LIU R, et al. Freestanding dual-gate oxide-based neuromorphic transistors for flexible artificial nociceptors. IEEE Transactions on Electron Devices, 2021, 68(1):415.
DOI URL |
| [25] |
FENG G, JIANG J, LI Y, et al. Flexible vertical photogating transistor network with an ultrashort channel for in‐sensor visual nociceptor. Advanced Functional Materials, 2021, 31(36):2104327.
DOI URL |
| [26] |
FORTUNATO E, BARQUINHA P, MARTINS R. Oxide semiconductor thin-film transistors: a review of recent advances. Advanced Materials, 2012, 24(22):2945.
DOI URL |
| [27] |
DENG X, WANG S Q, LIU Y X, et al. A flexible mott synaptic transistor for nociceptor simulation and neuromorphic computing. Advanced Functional Materials, 2021, 31(23):2101099.
DOI URL |
| [28] |
BARON R, MAIER C, ATTAL N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain, 2017, 158(2):261.
DOI PMID |
| [29] |
GU L, LI Y, XIE D, et al. Fully Optical-driving ionotronic InGaZnO4 phototransistor for gate-tunable bidirectional photofiltering and visual perception. IEEE Transactions on Electron Devices, 2022, 69(8):4382.
DOI URL |
| [30] |
LI M, YANG F S, HSU H C, et al. Defect engineering in ambipolar layered materials for mode-regulable nociceptor. Advanced Functional Materials, 2020, 31(5):2007587.
DOI URL |
| [31] |
VERRIPTIS M, CHANG P, FITZGERALD M, et al. The development of the nociceptive brain. Neuroscience, 2016, 338(3):207.
DOI PMID |
| [32] |
XIE D, JIANG J, DING L. Anisotropic 2D materials for post-Moore photoelectric devices. Journal of Semiconductors, 2022, 43(1):010201.
DOI |
| [33] |
LI Q, TAO Q, CHE Y, et al. Low voltage and robust InSe memristor using van der Waals electrodes integration. International Journal of Extreme Manufacturing, 2021, 3(4):045103.
DOI |
| [34] |
ZHAO Y, LIU W, ZHAO J, et al. The fabrication, characterization and functionalization in molecular electronics. International Journal of Extreme Manufacturing, 2022, 4(2):022003.
DOI |
| [35] | LI G XIE, D, ZHONG H, et al. Photo-induced non-volatile VO2 phase transition for neuromorphic ultraviolet sensors. Nature Communications, 2022, 13: 1729. |
| [1] | LIANG Yu, LIANG Ling-Yan, WU Wei-Hua, PEI Yu, YAO Zhi-Qiang, CAO Hong-Tao. Microfluidic-method-processed p-type NiOx Thin-film Transistors [J]. Journal of Inorganic Materials, 2019, 34(1): 79-84. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||