
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (7): 755-760.DOI: 10.15541/jim20180472
Previous Articles Next Articles
CHEN Chuan-Jie1,WEI Li-Geng1,WU Yue1,HU Cheng-Pei1,HU Yuan-Cong1,JIANG Jiu-Xin1,2,3( )
)
Received:2018-10-10
Revised:2018-12-03
Published:2019-07-20
Online:2019-06-26
Supported by:CLC Number:
CHEN Chuan-Jie, WEI Li-Geng, WU Yue, HU Cheng-Pei, HU Yuan-Cong, JIANG Jiu-Xin. Synthesis of Metastable Vaterite Phase of CaCO3 via Ethanol-calcium Method and Ethanol-water Binary Solvent Method[J]. Journal of Inorganic Materials, 2019, 34(7): 755-760.
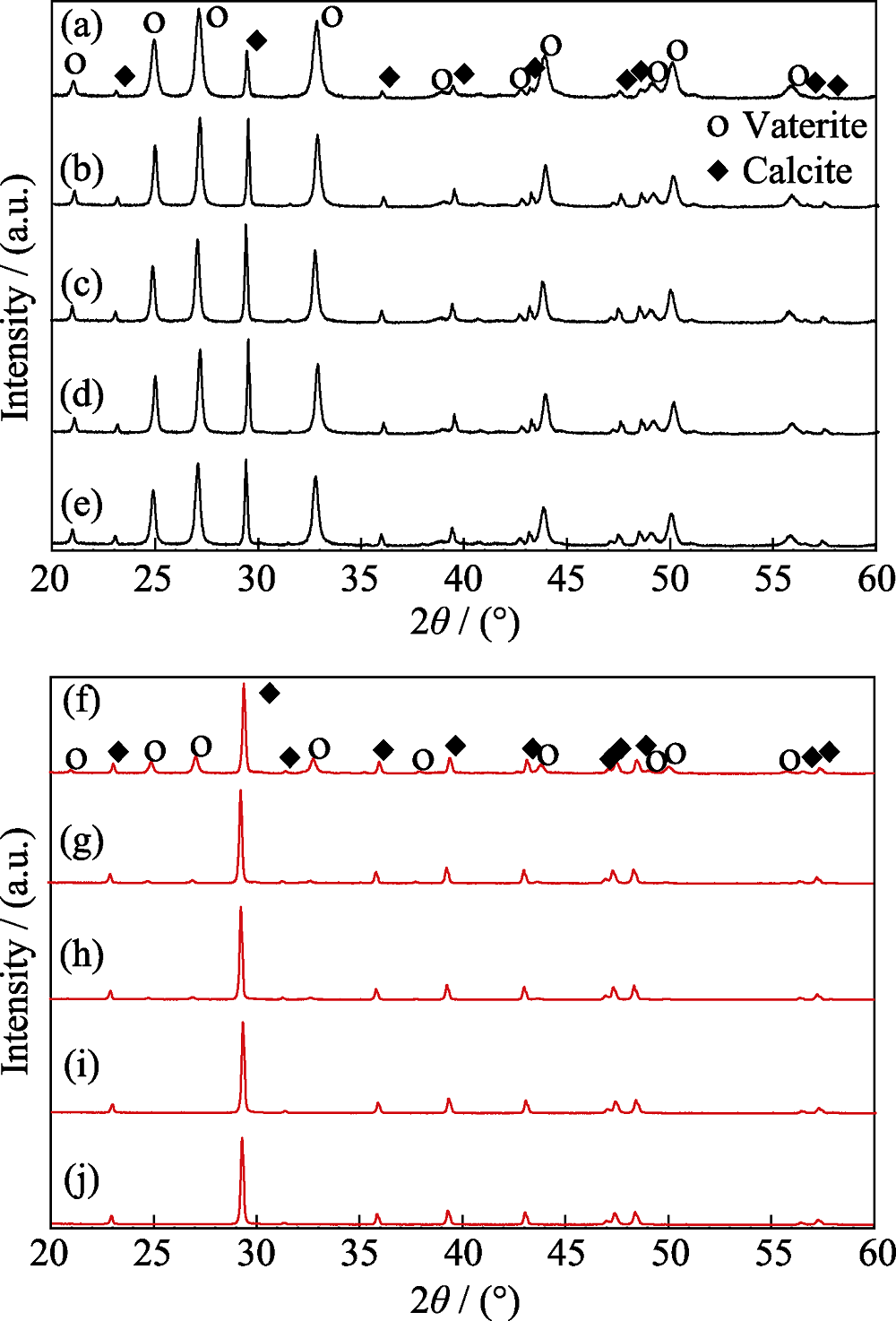
Fig. 1 XRD patterns of CaCO3 prepared from ethanol-calcium method (a-e) and EWBS method (f-j) after aging for different time (a, f) 0 min; (b, g) 20 min; (c, h) 90 min; (d, i) 22 h; (e, j) 42 h
| Aging time/min | Temperature/℃ | *Polymorphs/% | **Polymorphs/% | ||
|---|---|---|---|---|---|
| Vaterite | Calcite | Vaterite | Calcite | ||
| 0 | 20 | 90.4 | 9.6 | 46.5 | 53.5 |
| 20 | 20 | 84.4 | 15.6 | 12.3 | 87.7 |
| 90 | 20 | 83.5 | 15.9 | 10.5 | 89.5 |
| 1320 | 20 | 82.5 | 17.5 | 0 | 100 |
| 2520 | 20 | 81.4 | 18.6 | 0 | 100 |
| 60/10# | 0 | 85.8 | 14.2 | 57.1 | 42.9 |
| 60/10# | 20 | 84.1 | 15.9 | 44.3 | 55.7 |
| 60/10# | 40 | 73.3 | 26.7 | 22.6 | 77.4 |
| 60/10# | 60 | 70.2 | 29.8 | 0 | 100 |
| 60/10# | 80 | 64.8 | 35.2 | 0 | 100 |
Table 1 Phase compositions of CaCO3 prepared at different conditions
| Aging time/min | Temperature/℃ | *Polymorphs/% | **Polymorphs/% | ||
|---|---|---|---|---|---|
| Vaterite | Calcite | Vaterite | Calcite | ||
| 0 | 20 | 90.4 | 9.6 | 46.5 | 53.5 |
| 20 | 20 | 84.4 | 15.6 | 12.3 | 87.7 |
| 90 | 20 | 83.5 | 15.9 | 10.5 | 89.5 |
| 1320 | 20 | 82.5 | 17.5 | 0 | 100 |
| 2520 | 20 | 81.4 | 18.6 | 0 | 100 |
| 60/10# | 0 | 85.8 | 14.2 | 57.1 | 42.9 |
| 60/10# | 20 | 84.1 | 15.9 | 44.3 | 55.7 |
| 60/10# | 40 | 73.3 | 26.7 | 22.6 | 77.4 |
| 60/10# | 60 | 70.2 | 29.8 | 0 | 100 |
| 60/10# | 80 | 64.8 | 35.2 | 0 | 100 |
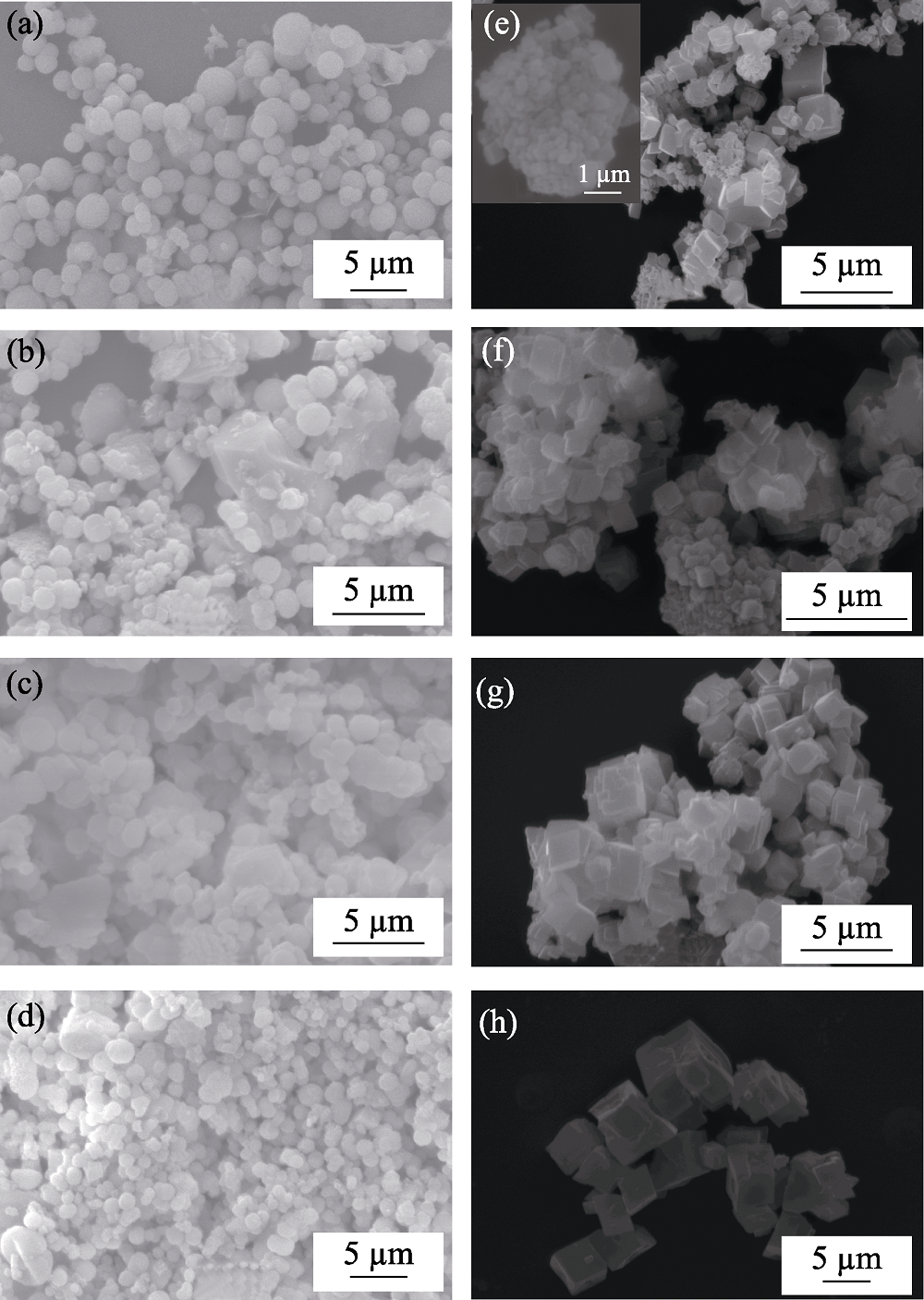
Fig. 2 SEM images of CaCO3 prepared from ethanol-calcium method (a-d) and EWBS method (e-h) after aging for different times (a, e) 0 min; (b, f) 20 min; (c, g) 90 min; (d, h) 42 h

Fig. 3 XRD patterns of CaCO3 prepared from ethanol-calcium method (a-e) and EWBS method (f-j) at different reaction temperatures (a, f) 0 min; (b, g) 20 ℃; (c, h) 40 ℃; (d, i) 60 ℃; (e, j) 80 ℃
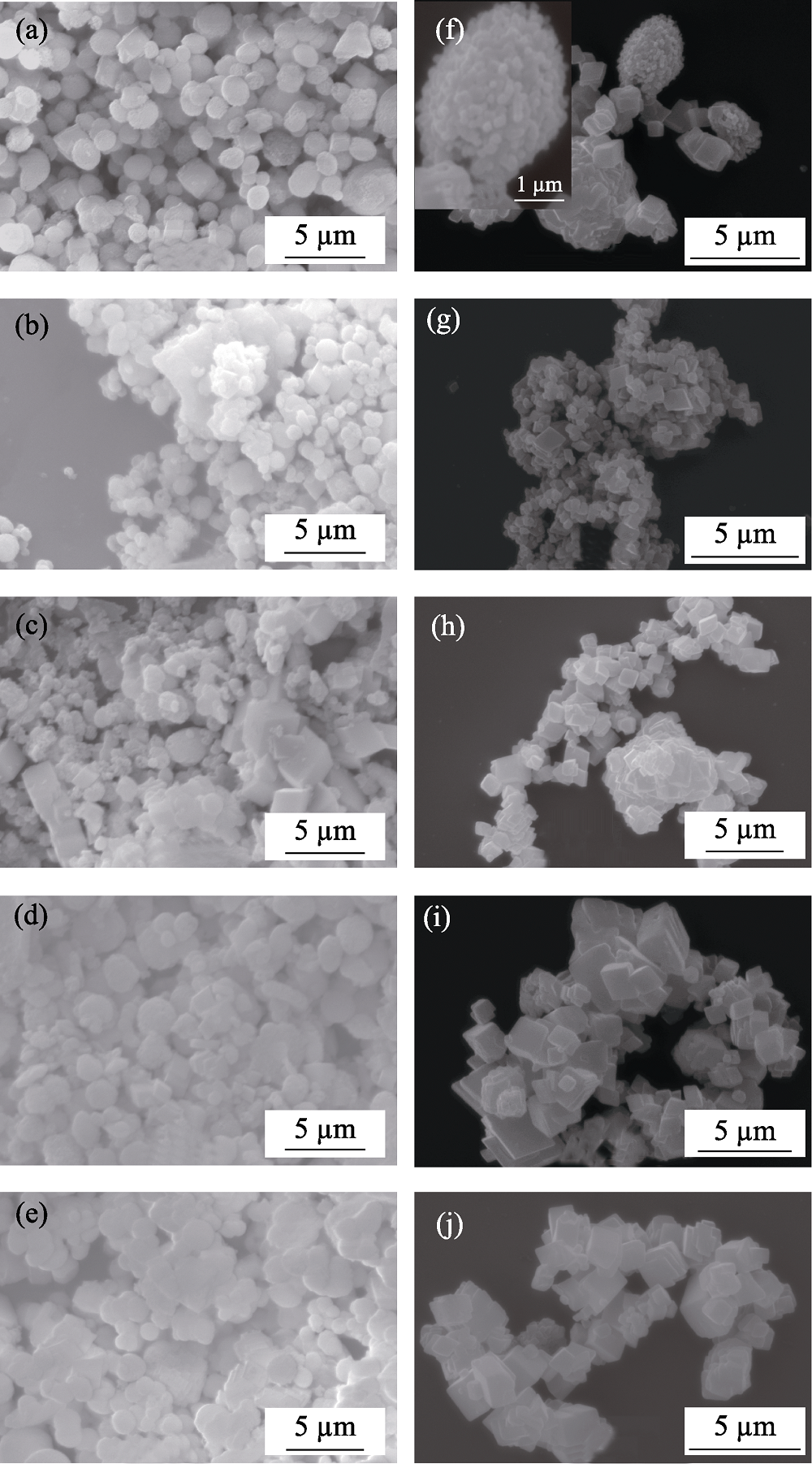
Fig. 4 SEM images of CaCO3 prepared from ethanol-calcium method (a-e) and EWBS method (f-j) at different reaction temperatures (a, f) 0 min; (b, g) 20 ℃; (c, h) 40 ℃; (d, i) 60 ℃; (e, j) 80 ℃
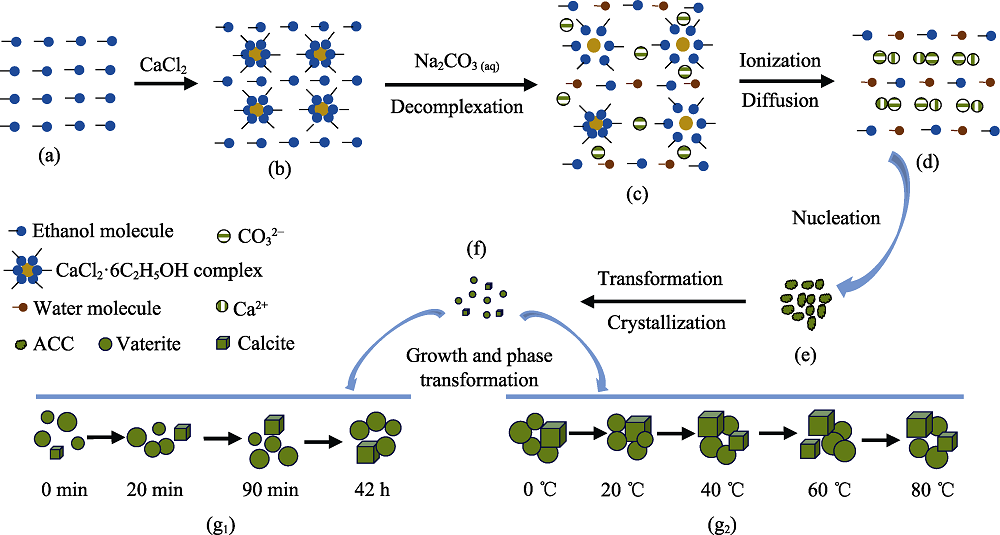
Fig. 5 The reaction process of ethanol-calcium method (a) Homogeneous anhydrous ethanol; (b) Formation of CaCl2·6C2H5OH complex and complex solution; (c) Decomplexation of CaCl2·6C2H5OH complex after the addition of Na2CO3 aqueous solution; (d) Ionization of CaCl2 in ethanol-aqueous solution and combination of Ca2+ and CO32-; (e) Formation of ACC; (f) Transformation of ACC to vaterite and calcite; (g) Growth of vaterite and calcite and phase transformation of vaterite to calcite at different aging time (g1) and different temperatures (g2)
| [1] |
TRUSHINA D B, BUKREEVA T V, KOVALCHUK M V , et al. CaCO3 vaterite microparticles for biomedical and personal care applications. Materials Science and Engineering C, 2014,45(1):644-658.
DOI URL |
| [2] |
NAKAMURA J, POOLOGASUNDARAMPILLAI G, JONES J R , et al. Tracking the formation of vaterite particles containing aminopropyl-functionalized silsesquioxane and their structure for bone regenerative medicine. Journal of Materials Chemistry B, 2013,1(35):4446-4454.
DOI URL |
| [3] |
QIU N, YIN H, JI B , et al. Calcium carbonate microspheres as carriers for the anticancer drug camptothecin. Materials Science and Engineering C, 2012,32(8):2634-2640.
DOI URL |
| [4] | SHA F, ZHU N, BAI Y J , et al. Controllable synthesis of various CaCO3 morphologies based on a CCUS idea. ACS Sustainable Chemistry & Engineering, 2016,4(6):3032-3044. |
| [5] |
ZOU J P, YANG H Z, XIAO P , et al. Controllable fabrication of calcium carbonate hollow microspheres with micro-nano hierarchical structure. Journal of Inorganic Materials, 2016,31(7):711-718.
DOI URL |
| [6] |
JIANG J Z, MA YX, ZHANG T , et al. Morphology and size control of calcium carbonate crystallized in a reverse micelle system with switchable surfactants. RSC Advances, 2015,5(98):80216-80219.
DOI URL |
| [7] |
LI Q, DING Y, LI F Q , et al. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. Journal of Crystal Growth, 2002,236(1/2/3):357-362.
DOI URL |
| [8] |
BEUVIER T, CALVIGNAC B, DELCROIX G J R , et al. Synthesis of hollow vaterite CaCO3 microspheres in supercritical carbon dioxide medium. Journal of Materials Chemistry, 2011,21(26):9757-9761.
DOI URL |
| [9] |
GUO Y M, WANG F F, ZHANG J , et al. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids. Research on Chemical Intermediates, 2013,39(6):2407-2415.
DOI URL |
| [10] |
JIANG J X, CHEN C J, XIAO B W , et al. Hierarchical CaCO3 particles self-assembled from metastable vaterite and stable calcite during the decomposition of Ca(HCO3)2. CrystEngComm, 2017,19(48):7332-7338.
DOI URL |
| [11] | LI Y S, LIU H R, XIA L L , et al. Fabrication and characterization of porous carbonate ceramic scaffolds. Journal of the Chinese Ceramic Society, 2014,42(7):851-856. |
| [12] | ZHANG L, YUE L H, WANG F , et al. Divisive effect of alcohol- water mixed solvents on growth morphology of calcium carbonate crystals. Journal of Materials Chemistry B, 2008,112(34):10668-10674. |
| [13] | LIU L, JIANG J, YU S H . Polymorph selection and structure evolution of CaCO3 mesocrystals under control of poly (sodium 4-styrenesulfonate): synergetic effect of temperature and mixed solvent. Crystal Growth & Design, 2014,14(11):6048-6056. |
| [14] |
HU Y, ZHOU Y, XU X , et al. Phase-controlled crystallization of amorphous calcium carbonate in ethanol-water binary solvents. Crystal Research and Technology, 2015,50(4):312-318.
DOI URL |
| [15] |
WANG A X, CHU D Q, WANG L M , et al. Preparation and characterization of novel spica-like hierarchical vaterite calcium carbonate and a hydrophilic poly (vinylidene fluoride)/calcium carbonate composite membrane. CrystEngComm, 2014,16(24):5198-5205.
DOI URL |
| [16] | MAO B G, CHU D Q, WANG A X , et al. Fabrication of flowerlike vaterite calcium carbonate crystal aggregates by self-assembly in water/ethanol mixtures. European Journal of Inorganic Chemistry, 2013(35):5958-5963. |
| [17] |
KOGA N, YAMANE Y, KIMURA T . Thermally induced transformations of calcium carbonate polymorphs precipitated selectively in ethanol/water solutions. Thermochimica Acta, 2011,512(1):13-21.
DOI URL |
| [18] | SAND K K RODRIGUEZ-BLANCO J D, MAKOVICKY E, , et al. Crystallization of CaCO3 in water-alcohol mixtures: spherulitic growth, polymorph stabilization, and morphology change. Crystal Growth & Design, 2011,12(2):842-853. |
| [19] |
JIN D, WANG F, YUE L . Phase and morphology evolution of vaterite crystals in water/ethanol binary solvent. Crystal Research and Technology, 2011,46(2):140-144.
DOI URL |
| [20] |
CHEN S F, YU S H, JIANG J , et al. Polymorph discrimination of CaCO3 mineral in an ethanol/water solution: formation of complex vaterite superstructures and aragonite rods. Chemistry of Materials, 2006,18(1):115-122.
DOI URL |
| [21] |
KONTOYANNIS C G, VAGENAS N V . Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst, 2000,125(2):251-255.
DOI URL |
| [22] |
WEI H, SHEN Q, ZHAO Y , et al. Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite. Journal of Crystal Growth, 2003,250(3):516-524.
DOI URL |
| [23] |
FLATEN E M, SEIERSTEN M, ANDREASSEN J P , et al. Polymorphism and morphology of calcium carbonate precipitated in mixed solvents of ethylene glycol and water. Journal of Crystal Growth, 2009,311(13):3533-3538.
DOI URL |
| [24] |
CHEN J, XIANG L . Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technology, 2009,189(1):64-69.
DOI URL |
| [25] | JIANG J, CHEN S F, LIU L , et al. Template-free polymorph discrimination and synthesis of calcium carbonate minerals. Chemical Communications, 2009(39):5853-5855. |
| [26] |
XIE A J, SHEN Y H, ZHANG C Y , et al. Crystal growth of calcium carbonate with various morphologies in different amino acid systems. Journal of Crystal Growth, 2005,285(3):436-443.
DOI URL |
| [27] | GENG X, LIU L, JIANG J , et al. Crystallization of CaCO3 mesocrystals and complex aggregates in a mixed solvent media using polystyrene sulfonate as a crystal growth modifier. Crystal Growth & Design, 2010,10(8):3448-3453. |
| [28] | HAWLICKA E, SWIATLA-WOJCIK D . MD simulation studies of selective solvation in methanol-water mixtures: an effect of the charge density of a solute. Journal of Materials Chemistry A, 2002,106(7):1336-1345. |
| [29] |
HAN Y S, HADIKO G, FUJI M , et al. Factors affecting the phase and morphology of CaCO3 prepared by a bubbling method. Journal of the European Ceramic Society, 2006,26(4):843-847.
DOI URL |
| [1] | CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion [J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282. |
| [2] | LIU Ziyang, GENG Zhen, LI Zhaoyang. Preparing Biomedical CaCO3/HA Composite with Oyster Shell [J]. Journal of Inorganic Materials, 2020, 35(5): 601-607. |
| [3] | DU Xudong, TANG Chengyuan, YANG Xiaoli, CHENG Jianbo, JIA Yuke, YANG Shubin. High-efficiency Biogenic Calcium Carbonate for Adsorption of Pb(II) and Methyl Orange from Wastewater [J]. Journal of Inorganic Materials, 2020, 35(3): 315-323. |
| [4] | JIANG Jiu-Xin, WU Yue, HE Yao, GAO Song, ZHANG Chen, SHEN Tong, LIU Jia-Ning. Progress in Tuning of Metastable Vaterite Calcium Carbonate [J]. Journal of Inorganic Materials, 2017, 32(7): 681-690. |
| [5] | ZOU Jian-Peng, YANG Hong-Zhi, XIAO Ping, PAN Yi-Feng. Controllable Fabrication of Calcium Carbonate Hollow Microspheres with Micro-nano Hierarchical Structure [J]. Journal of Inorganic Materials, 2016, 31(7): 711-718. |
| [6] | HU Zhi-Bo, YAN Yang, ZHENG Shui-Lin, SUN Qin, YIN Sheng-Nan. Preparation and Characterization of Humidity Control Material Based on Diatomite/Ground Calcium Carbonate Composite [J]. Journal of Inorganic Materials, 2016, 31(1): 81-87. |
| [7] | WANG Kun, KANG Li-Tao, CHEN Shi, DONG Li, LIANG Wei, GAO Feng. Effects of Reaction Temperature on Structure and Optical Properties of Hydrothermally Prepared (NH4)xWO3-y and WO3·1/3H2O [J]. Journal of Inorganic Materials, 2014, 29(5): 550-556. |
| [8] | ZHOU Chao, FENG Qing, GAO Yan-Min. Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Powders by Solvothermal Method [J]. Journal of Inorganic Materials, 2014, 29(5): 487-492. |
| [9] | YANG Ya-Nan, ZHU Xiao-Li, KONG Xiang-Zheng. Controls of Crystal Morphology, Size and Structure in Spontaneous Precipitation of Calcium Carbonate [J]. Journal of Inorganic Materials, 2013, 28(12): 1313-1320. |
| [10] | MA Yu-Fei, QIAO Li, FENG Qing-Ling. Research Progress on Biomineralization Mechanism of Freshwater Pearl [J]. Journal of Inorganic Materials, 2013, 28(1): 109-116. |
| [11] | MA Jun, LIU Hua-Yan, CHEN Yin-Fei. Reuse of MgCl2 as Crystal Controlling Agent in the Synthesis Process of Needle-like Calcium Carbonate [J]. Journal of Inorganic Materials, 2011, 26(11): 1199-1204. |
| [12] | SUN Jing, XIAO Yu-Mei, FAN Hong-Song, ZHANG Xing-Dong. Effect of Hydroxyapatite on the Preparation and Properties of Alginate Hydrogel [J]. Journal of Inorganic Materials, 2010, 25(10): 1087-1091. |
| [13] | WEN Yan,XIANG Lan,JIN Yong. Preparation of Plate-like Calcium Carbonate [J]. Journal of Inorganic Materials, 2002, 17(6): 1315-1320. |
| [14] | YANG Qing-Feng,GU An-Zhong,LIU Yang-Qiao,ZENG Hua-Rong,DING Jie,SHEN Zi-Qiu. Effects of PAA and PBTCA on CaCO3 Micro-Structure [J]. Journal of Inorganic Materials, 2002, 17(3): 559-565. |
| [15] | XU Wang-Sheng,HE Bing-Zhong,JIN Shi-Wei,XUAN Ai-Guo. Preparation of Nanometer Calcium Carbonate by Multistage Spray Carbonation [J]. Journal of Inorganic Materials, 2001, 16(5): 985-988. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||