无机材料学报 ›› 2025, Vol. 40 ›› Issue (1): 53-60.DOI: 10.15541/jim20240140 CSTR: 32189.14.10.15541/jim20240140
所属专题: 【能源环境】氢能材料(202506)
连敏丽( ), 苏佳欣, 黄鸿杨, 嵇玉寅, 邓海帆, 张彤, 陈崇启, 李达林(
), 苏佳欣, 黄鸿杨, 嵇玉寅, 邓海帆, 张彤, 陈崇启, 李达林( )
)
收稿日期:2024-03-22
修回日期:2024-07-19
出版日期:2025-01-20
网络出版日期:2024-07-26
通讯作者:
李达林, 研究员. E-mail: dalinli@fzu.edu.cn作者简介:连敏丽(1999-), 女, 硕士研究生. E-mail: 975216049@qq.com
基金资助:
LIAN Minli( ), SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin(
), SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin( )
)
Received:2024-03-22
Revised:2024-07-19
Published:2025-01-20
Online:2024-07-26
Contact:
LI Dalin, professor. E-mail: dalinli@fzu.edu.cnAbout author:LIAN Minli (1999-), female, Master candidate. E-mail: 975216049@qq.com
Supported by:摘要:
氨分解作为一种很有前景的现场制氢技术, 关键在于开发出廉价、高性能的催化剂。本研究通过共沉淀法合成系列NixMg75-xAl25类水滑石化合物(HTlc)作为前驱体, 经过焙烧和还原处理制备负载型高分散Ni/Mg(Al)O催化剂并用于氨分解制氢, 采用不同研究手段对样品进行了表征, 考察了Ni含量和氨还原对催化性能的影响。结果显示, HTlc前驱体经过焙烧分解形成Mg(Ni, Al)O固溶体, Ni物种与载体之间存在较强的相互作用, 经750 ℃氨还原得到高分散Ni金属纳米颗粒, 其平均晶粒尺寸为5.9~7.7 nm。质谱分析表明, 氨还原过程中无氮氧化物(NOx)生成, 同时750 ℃氨还原与氢还原催化剂的活性相当, 说明氨是一种合适的还原气。催化剂活性随着Ni含量和还原温度升高而增加。其中, 750 ℃氨还原Ni20Mg55Al25催化剂在30000 mL·gcat-1·h-1、600 ℃下的氨转化率为98%, 且在100 h反应过程中转化率保持不变, Ni金属无明显烧结现象, 催化剂表现出良好的活性、稳定性和抗烧结性能。
中图分类号:
连敏丽, 苏佳欣, 黄鸿杨, 嵇玉寅, 邓海帆, 张彤, 陈崇启, 李达林. Ni-Mg-Al类水滑石衍生镍基催化剂的制备及其氨分解性能[J]. 无机材料学报, 2025, 40(1): 53-60.
LIAN Minli, SU Jiaxin, HUANG Hongyang, JI Yuyin, DENG Haifan, ZHANG Tong, CHEN Chongqi, LI Dalin. Supported Ni Catalysts from Ni-Mg-Al Hydrotalcite-like Compounds:Preparation and Catalytic Performance for Ammonia Decomposition[J]. Journal of Inorganic Materials, 2025, 40(1): 53-60.

图1 NixMg75-xAl25 HTlc前驱体的(A)XRD谱图和(B)SEM照片
Fig. 1 (A) XRD patterns and (B) SEM images of the as-synthesized NixMg75-xAl25 HTlc precursors (a) Ni5Mg70Al25-HTlc; (b) Ni10Mg65Al25-HTlc; (c) Ni15Mg60Al25-HTlc; (d) Ni20Mg55Al25-HTlc; (e) Ni25Mg50Al25-HTlc
| Sample | Metal content/(%, in atom) | Lattice parameter/nm | |||
|---|---|---|---|---|---|
| Ni | Mg | Al | a | c | |
| Ni5Mg70Al25-HTlc | 5.5 | 68.1 | 26.4 | 0.306 | 2.344 |
| Ni10Mg65Al25-HTlc | 10.4 | 63.1 | 26.5 | 0.305 | 2.344 |
| Ni15Mg60Al25-HTlc | 15.9 | 58.4 | 25.7 | 0.306 | 2.344 |
| Ni20Mg55Al25-HTlc | 20.5 | 53.5 | 26.0 | 0.305 | 2.344 |
| Ni25Mg50Al25-HTlc | 25.6 | 48.9 | 25.5 | 0.305 | 2.347 |
表1 NixMg75-xAl25 HTlc前驱体的金属含量和晶格参数
Table 1 Metal contents and lattice parameters of the NixMg75-xAl25 HTlc precursors
| Sample | Metal content/(%, in atom) | Lattice parameter/nm | |||
|---|---|---|---|---|---|
| Ni | Mg | Al | a | c | |
| Ni5Mg70Al25-HTlc | 5.5 | 68.1 | 26.4 | 0.306 | 2.344 |
| Ni10Mg65Al25-HTlc | 10.4 | 63.1 | 26.5 | 0.305 | 2.344 |
| Ni15Mg60Al25-HTlc | 15.9 | 58.4 | 25.7 | 0.306 | 2.344 |
| Ni20Mg55Al25-HTlc | 20.5 | 53.5 | 26.0 | 0.305 | 2.344 |
| Ni25Mg50Al25-HTlc | 25.6 | 48.9 | 25.5 | 0.305 | 2.347 |
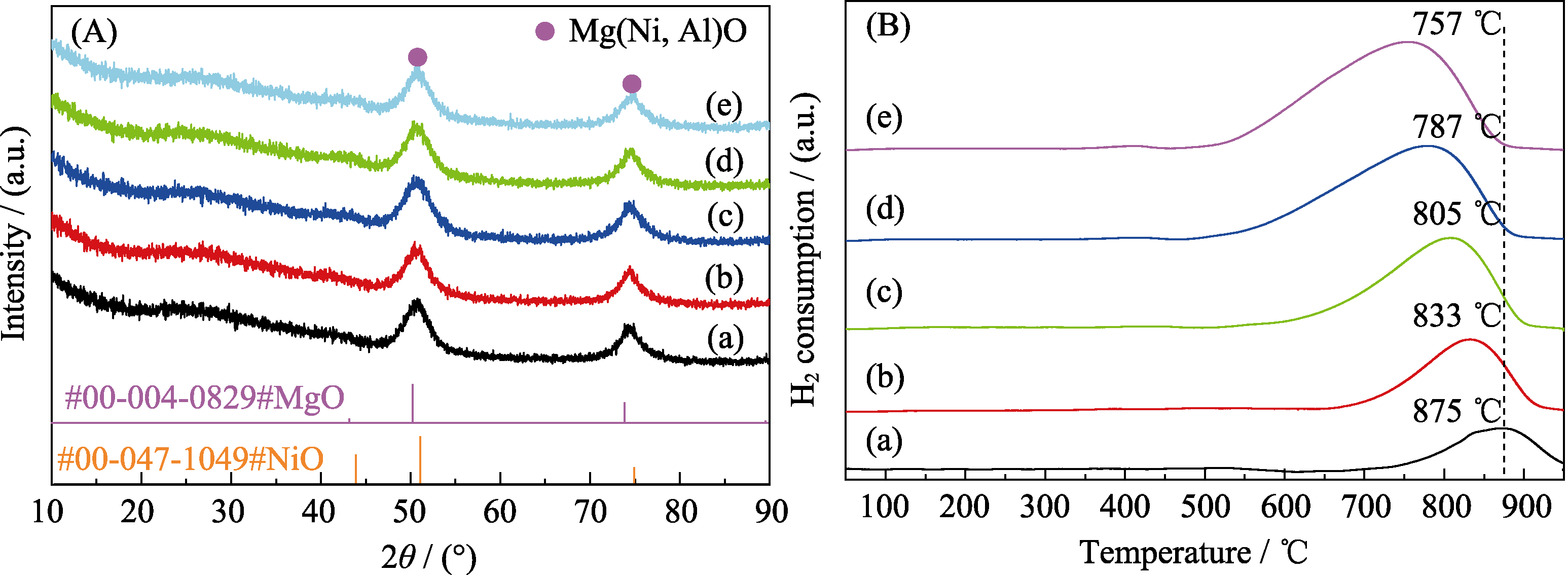
图2 NixMg75-xAl25焙烧样品的(A)XRD谱图和(B)H2-TPR曲线
Fig. 2 (A) XRD patterns and (B) H2-TPR profiles of calcined NixMg75-xAl25 (a) Ni5Mg70Al25; (b) Ni10Mg65Al25; (c) Ni15Mg60Al25; (d) Ni20Mg55Al25; (e) Ni25Mg50Al25
| Sample | Surface area/ (m2·gcat-1) | Pore volume/ (cm3·gcat-1) | Pore diameter/ nm | Ni crystal size/ nm | |
|---|---|---|---|---|---|
| Ni5Mg70Al25 | 197.3 | 0.45 | 7.5 | 7.7 | |
| Ni10Mg65Al25 | 173.0 | 0.31 | 5.6 | 7.6 | |
| Ni15Mg60Al25 | 217.4 | 0.36 | 5.6 | 5.9 | |
| Ni20Mg55Al25 | 218.6 | 0.38 | 5.5 | 5.9 | |
| Ni25Mg50Al25 | 204.4 | 0.43 | 7.0 | 6.7 | |
表2 焙烧样品的织构性质和还原催化剂中Ni金属晶粒尺寸
Table 2 Textural properties of the calcined samples and crystallite size of Ni metal in the reduced catalysts
| Sample | Surface area/ (m2·gcat-1) | Pore volume/ (cm3·gcat-1) | Pore diameter/ nm | Ni crystal size/ nm | |
|---|---|---|---|---|---|
| Ni5Mg70Al25 | 197.3 | 0.45 | 7.5 | 7.7 | |
| Ni10Mg65Al25 | 173.0 | 0.31 | 5.6 | 7.6 | |
| Ni15Mg60Al25 | 217.4 | 0.36 | 5.6 | 5.9 | |
| Ni20Mg55Al25 | 218.6 | 0.38 | 5.5 | 5.9 | |
| Ni25Mg50Al25 | 204.4 | 0.43 | 7.0 | 6.7 | |
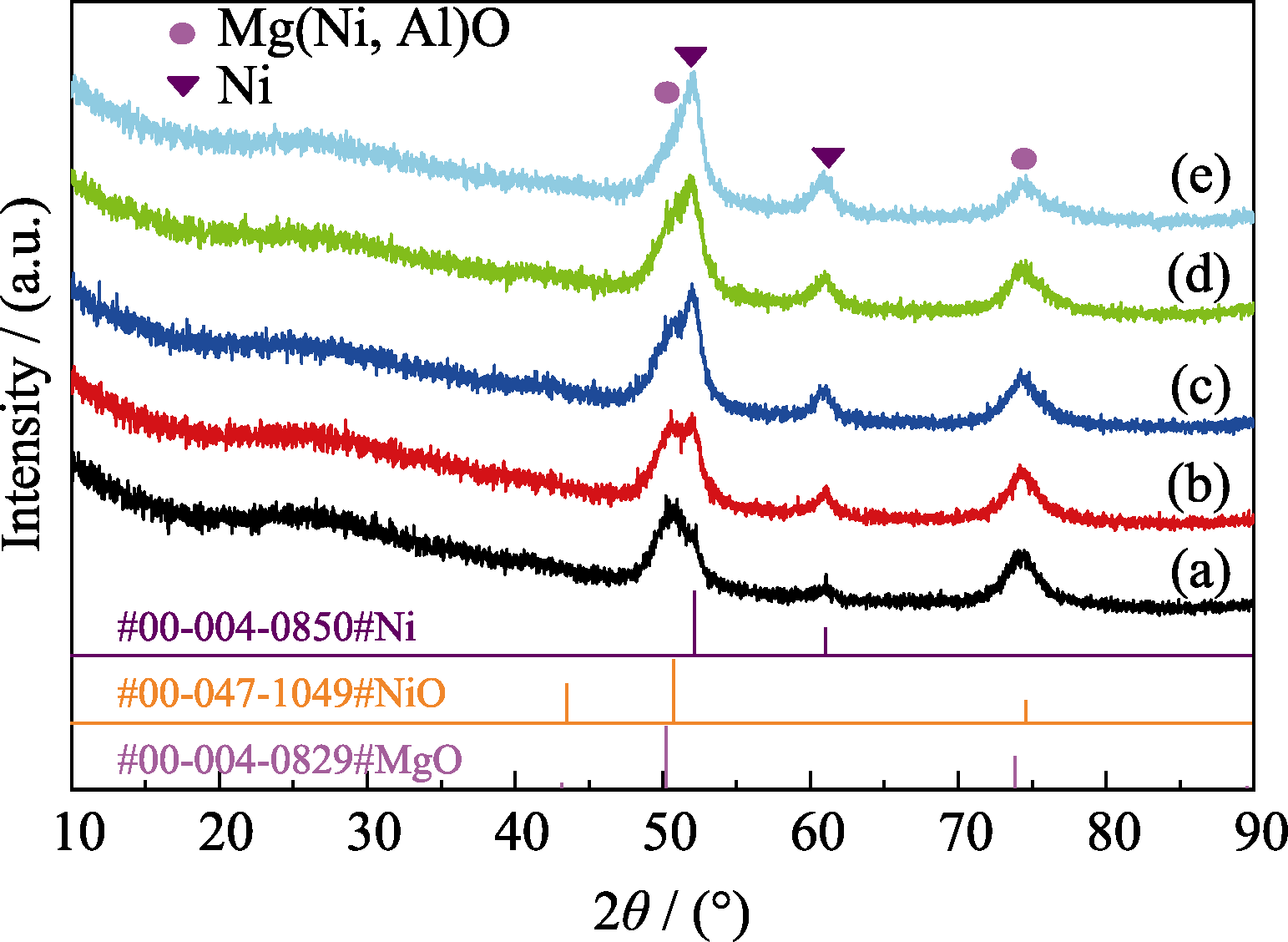
图4 750 ℃氨还原NixMg75-xAl25催化剂的XRD谱图
Fig. 4 XRD patterns of NixMg75-xAl25 catalysts reduced at 750 ℃ with NH3 (a) Ni5Mg70Al25; (b) Ni10Mg65Al25; (c) Ni15Mg60Al25; (d) Ni20Mg55Al25; (e) Ni25Mg50Al25

图5 750 ℃氨还原Ni20Mg55Al25催化剂的(a)HAADF-STEM照片、(b~f)EDX元素分布图、(g, h)TEM和HRTEM照片、(i)Ni金属粒径分布图
Fig. 5 (a) HAADF-STEM image, (b-f) EDX elemental mappings, (g) TEM image, (h) HRTEM image, and (i) Ni particle size distribution for the Ni20Mg55Al25 catalyst reduced at 750 ℃ with NH3
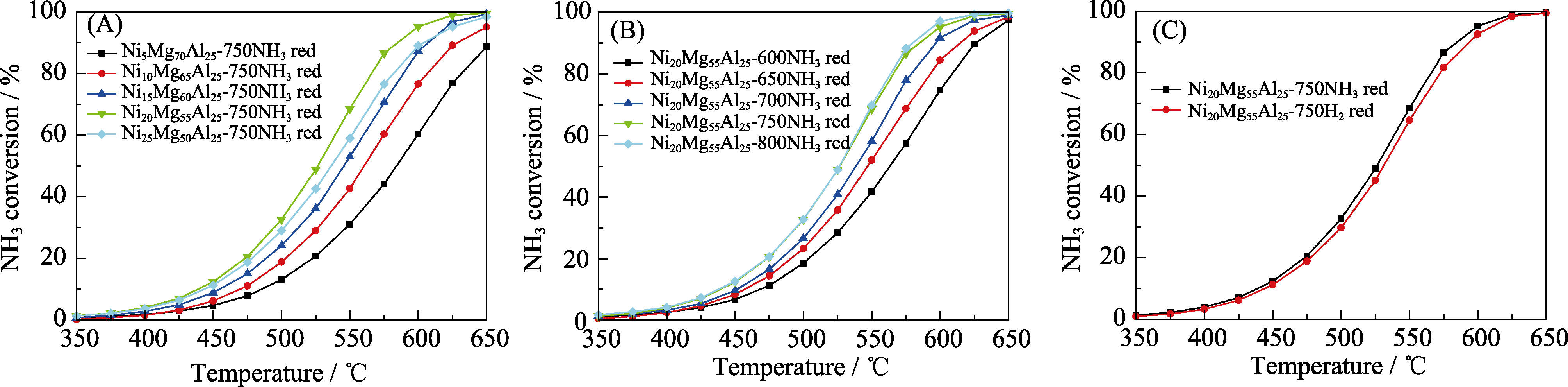
图6 NixMg75-xAl25催化剂的氨分解活性
Fig. 6 Catalytic ammonia decomposition over the NixMg75-xAl25 catalysts Influence of (A) Ni contents, (B) reduction temperature, and (C) reduction atmosphere

图7 Ni20Mg55Al25催化剂(A)在不同空速下的活性、(B)长期稳定性和(C)反应后XRD谱图
Fig. 7 (A) Catalytic activities at different space velocities and (B) long-term stability of Ni20Mg55Al25 (pre-reduced with NH3 at 750 ℃), and (C) XRD patterns of the fresh and used Ni20Mg55Al25
| Catalyst | Feed gas | Space velocity/ (mL·gcat-1·h-1) | Temperature/ ℃ | NH3 conversion/ % | NH3 reaction rate/ (mmol· gcat-1·min-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni20Mg55Al25 | Pure NH3 | 30000 | 550 | 68.5 | 15.3 | This work |
| NiCe0.85Zr0.15O | Pure NH3 | 30000 | 550 | 63 | 14.1 | [ |
| Ni/cCeO2(IMP) | Pure NH3 | 30000 | 550 | 51 | 11.4 | [ |
| Ni_MgAl(6:1) | Pure NH3 | 30000 | 550 | 48 | 10.7 | [ |
| Ni/MCM-41(TIE) | Pure NH3 | 30000 | 550 | 47.6 | 10.6 | [ |
| Ni/BaZrO3 | Pure NH3 | 20000 | 550 | 62 | 9.2 | [ |
| Ni1.2Ce0.1Al | Pure NH3 | 45000 | 550 | 26 | 8.7 | [ |
| Ni/La2O3 | Pure NH3 | 6000 | 550 | 78.9 | 3.5 | [ |
表3 Ni催化剂的氨分解性能
Table 3 Catalytic performance of Ni catalysts for ammonia decomposition
| Catalyst | Feed gas | Space velocity/ (mL·gcat-1·h-1) | Temperature/ ℃ | NH3 conversion/ % | NH3 reaction rate/ (mmol· gcat-1·min-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni20Mg55Al25 | Pure NH3 | 30000 | 550 | 68.5 | 15.3 | This work |
| NiCe0.85Zr0.15O | Pure NH3 | 30000 | 550 | 63 | 14.1 | [ |
| Ni/cCeO2(IMP) | Pure NH3 | 30000 | 550 | 51 | 11.4 | [ |
| Ni_MgAl(6:1) | Pure NH3 | 30000 | 550 | 48 | 10.7 | [ |
| Ni/MCM-41(TIE) | Pure NH3 | 30000 | 550 | 47.6 | 10.6 | [ |
| Ni/BaZrO3 | Pure NH3 | 20000 | 550 | 62 | 9.2 | [ |
| Ni1.2Ce0.1Al | Pure NH3 | 45000 | 550 | 26 | 8.7 | [ |
| Ni/La2O3 | Pure NH3 | 6000 | 550 | 78.9 | 3.5 | [ |
| [1] | LAN R, IRVINE J T S, TAO S. Ammonia and related chemicals as potential indirect hydrogen storage materials. International Journal of Hydrogen Energy, 2012, 37(2): 1482. |
| [2] | SCHUTH F, PALKOVITS R, SCHLOGL R, et al. Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy & Environmental Science, 2012, 5(4): 6278. |
| [3] | WAN Z, TAO Y, SHAO J, et al. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Conversion and Management, 2021, 228: 113729. |
| [4] | 王晓光, 韦永德, 张建, 等. 高效镍基氨分解催化体系中载体作用的研究. 石油学报(石油加工), 2006, 22(5): 33. |
| [5] | MAEDA A, HU Z, KUNIMORI K, et al. Effect of high- temperature reduction on ammonia decomposition over niobia- supported and niobia-promoted rhodium catalysts. Catalysis Letters, 1988, 1(5): 155. |
| [6] | JU X, LIU L, YU P, et al. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Applied Catalysis B: Environmental, 2017, 211: 167. |
| [7] | 倪平, 储伟, 罗仕忠, 等. 钡修饰Ir/SiO2催化剂对氨分解促进作用的研究. 合成化学, 2007, 15(4): 407. |
| [8] | PARKER L A, CARTER J H, DUMMER N F, et al. Ammonia decomposition enhancement by Cs-promoted Fe/Al2O3 catalysts. Catalysis Letters, 2020, 150(12): 3369. |
| [9] | MALEKI H, BERTOLA V. Co-Ce-Al-O mesoporous catalysts for hydrogen generation via ammonia decomposition. International Journal of Hydrogen Energy, 2024, 51: 267. |
| [10] | XU J, YAN H, JIN Z, et al. Facile synthesis of stable MO2N nanobelts with high catalytic activity for ammonia decomposition. Chinese Journal of Chemistry, 2019, 37(4): 364. |
| [11] | LI L, WU J, SHAO J, et al. Impacts of SiO2 shell structure of Ni@SiO2 nanocatalysts on their performance for catalytic decomposition of ammonia. Catalysis Letters, 2016, 147(1): 141. |
| [12] | SIMONSEN S B, CHAKRABORTY D, CHORKENDORFF I, et al. Alloyed Ni-Fe nanoparticles as catalysts for NH3 decomposition. Applied Catalysis A: General, 2012, 447/448: 22. |
| [13] | ZANMAN S F, JOLAOSO L A, PODILA S, et al. Ammonia decomposition over citric acid chelated γ-Mo2N and Ni2Mo3N catalysts. International Journal of Hydrogen Energy, 2018, 43(36): 17252. |
| [14] | HE H, JIANG H, YANG F, et al. Bimetallic NixCo10-x/CeO2 as highly active catalysts to enhance mid-temperature ammonia decomposition: kinetics and synergies. International Journal of Hydrogen Energy, 2023, 48(13): 5030. |
| [15] | PODILA S, DRISS H, ZAMAN S F, et al. MgFe and Mg-Co-Fe mixed oxides derived from hydrotalcites: highly efficient catalysts for COx free hydrogen production from NH3. International Journal of Hydrogen Energy, 2020, 45(1): 873. |
| [16] | PANSARE S S, TORRES W, GOODWIN J G. Ammonia decomposition on tungsten carbide. Catalysis Communications, 2007, 8(4): 649. |
| [17] | CHOI J G. Ammonia decomposition over vanadium carbide catalysts. Journal of Catalysis, 1999, 182(1): 104. |
| [18] | OTREMBA T, FRENZEL N, LERCH M, et al. Kinetic studies on ammonia decomposition over zirconium oxynitride. Applied Catalysis A: General, 2011, 392(1): 103. |
| [19] | LE T A, DO Q C, KIM Y, et al. A review on the recent developments of ruthenium and nickel catalysts for COx-free H2 generation by ammonia decomposition. Korean Journal of Chemical Engineering, 2021, 38(6): 1087. |
| [20] | TAKEHIRA K. Recent development of layered double hydroxide- derived catalysts—rehydration, reconstitution, and supporting, aiming at commercial application. Applied Clay Science, 2017, 136: 112. |
| [21] | TAKEHIRA K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg-Al hydrotalcite-like anionic clay. Journal of Catalysis, 2004, 221(1): 43. |
| [22] | SERRANOL A, RODRIGUEZ L, MUNOZ G, et al. Biogas reforming on La-promoted NiMgAl catalysts derived from hydrotalcite-like precursors. Journal of Power Sources, 2011, 196(9): 4404. |
| [23] | BETCHAKU M, NAKAGAWA Y, TAMURA M, et al. Combination of hydrotalcite-like-compound-derived Ni-Fe/Mg/Al and ceria- supported Rh catalysts for fuel reforming in exhaust gas recirculation system of gasoline engine. Fuel Processing Technology, 2022, 225: 107061. |
| [24] | YU X P, CHU W, WANG N, et al. Hydrogen production by ethanol steam reforming on NiCuMgAl catalysts derived from hydrotalcite-like precursors. Catalysis Letters, 2011, 141(8): 1228. |
| [25] | DENG L, LIN H, LIU X, et al. Nickel nanoparticles derived from the direct thermal reduction of Ni-containing Ca-Al layered double hydroxides for hydrogen generation via ammonia decomposition. International Journal of Hydrogen Energy, 2021, 46(77): 38351. |
| [26] | SATO K, ABE N, KAWAGOE T, et al. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. International Journal of Hydrogen Energy, 2017, 42(10): 6610. |
| [27] | SU Q, GU L, YAO Y, et al. Layered double hydroxides derived Nix(MgyAlzOn) catalysts: enhanced ammonia decomposition by hydrogen spillover effect. Applied Catalysis B: Environmental, 2017, 201: 451. |
| [28] | OLSBYE U, AKPORIAYE D, RYTTER E, et al. On the stability of mixed M2+/M3+ oxides. Applied Catalysis A: General, 2002, 224(1): 39. |
| [29] | OKURA K, MIYAZAKI K, MUROYAMA H, et al. Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen. RSC Advances, 2018, 8(56): 32102. |
| [30] | MUROYAMA H, SABURI C, MATSUI T, et al. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Applied Catalysis A: General, 2012, 443/444: 119. |
| [31] | LI X, JI W, ZHAO J, et al.Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. Journal of Catalysis, 2005, 236(2): 181. |
| [32] | ZHENG W, ZHANG J, GE Q, et al. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Applied Catalysis B: Environmental, 2008, 80(1/2): 98. |
| [33] | WU K, CAO C F, ZHOU C, et al. Engineering of Ce3+-O-Ni structures enriched with oxygen vacancies via Zr doping for effective generation of hydrogen from ammonia. Chemical Engineering Science, 2021, 245: 116818. |
| [34] | LIU H, ZHANG Y, LIU S, et al. Ni-CeO2 nanocomposite with enhanced metal-support interaction for effective ammonia decomposition to hydrogen. Chemical Engineering Journal, 2023, 473: 145371. |
| [1] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [2] | 于泽龙, 唐春, 饶家豪, 郭恒, 周莹. 碱性电解水大电流密度电催化剂的制备及经济性研究[J]. 无机材料学报, 2025, 40(12): 1405-1413. |
| [3] | 唐阳, 刘立敏, 周晓亮, 张搏, 蒋星洲, 贾浩义, 罗延麟庆. 质子陶瓷膜反应器的制备及低温氨分解性能研究[J]. 无机材料学报, 2025, 40(11): 1277-1284. |
| [4] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [5] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [6] | 马慧, 陶疆辉, 王艳妮, 韩玉, 王亚斌, 丁秀萍. 硅钛杂化介孔球负载金纳米粒子及其催化性能调控[J]. 无机材料学报, 2022, 37(4): 404-412. |
| [7] | 邓霁峰, 陈顺鹏, 武晓娟, 郑捷, 李星国. 水解制氢材料研究进展[J]. 无机材料学报, 2021, 36(1): 1-8. |
| [8] | 王苹,李心宇,时占领,李海涛. Ag与Ag2O协同增强TiO2光催化制氢性能的研究[J]. 无机材料学报, 2020, 35(7): 781-788. |
| [9] | 魏磊, 马麦霞, 卢艳红, 王东升, 张素玲, 赵娣, 马卫攀. 酵母菌模板辅助合成Co3O4空心微球催化NaBH4水解制氢[J]. 无机材料学报, 2018, 33(6): 648-652. |
| [10] | 王松灿, 汤枫秋, 王连洲. 光电催化分解水用可见光响应型氧化物光阳极的改性研究进展[J]. 无机材料学报, 2018, 33(2): 173-197. |
| [11] | 马雅婷, 李巧玲. TiO2/Co3O4复合纳米颗粒的制备及其光催化制氢性能[J]. 无机材料学报, 2016, 31(8): 841-844. |
| [12] | 魏 婕, 李雪冬, 王宏志, 张青红, 李耀刚. NCDs/TiO2复合材料的制备及其在太阳光下催化制氢的应用[J]. 无机材料学报, 2015, 30(9): 925-930. |
| [13] | 桑换新, 田 野, 王希涛, 陶 磊. Bi掺杂纳米TiO2光催化甘油水溶液制氢性能研究[J]. 无机材料学报, 2012, 27(12): 1283-1288. |
| [14] | 于 波, 张文强, 梁明德, 张 平, 徐景明. PMMA造孔剂对固体氧化物电解池制氢性能的影响[J]. 无机材料学报, 2011, 26(8): 807-812. |
| [15] | 张耀君,张 莉. 复合材料CdS/Al-HMS的制备及可见光催化降解污染物制氢活性研究[J]. 无机材料学报, 2008, 23(1): 66-70. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||