无机材料学报 ›› 2022, Vol. 37 ›› Issue (10): 1093-1101.DOI: 10.15541/jim20220027 CSTR: 32189.14.10.15541/jim20220027
收稿日期:2022-01-17
修回日期:2022-04-18
出版日期:2022-10-20
网络出版日期:2022-04-26
通讯作者:
周晓霞, 副研究员. E-mail: zhouxiaoxia@mail.sic.ac.cn作者简介:李成金 (1995-), 男, 硕士研究生. E-mail: ChengjinLL@163.com
基金资助:
LI Chengjin1,2( ), XUE Yi1,2, ZHOU Xiaoxia2(
), XUE Yi1,2, ZHOU Xiaoxia2( ), CHEN Hangrong2
), CHEN Hangrong2
Received:2022-01-17
Revised:2022-04-18
Published:2022-10-20
Online:2022-04-26
Contact:
ZHOU Xiaoxia, associate professor. E-mail: zhouxiaoxia@mail.sic.ac.cnAbout author:LI Chengjin (1995-), male, Master candidate. E-mail: ChengjinLL@163.com
Supported by:摘要:
将CO2转化为高附加值的化学品是实现碳循环, 缓解能源危机和环境问题的有效途径之一。金属与半导体复合电极, 利用光电耦合技术为CO2转化提供了一种新思路。本研究通过电沉积的方法在碱刻蚀处理后的Si片上制备了双金属Bi、Zn共修饰的Si基光电阴极(BiZnx/Si), 用于CO2的光电催化还原。研究表明, 引入金属Bi和Zn能够改善光的吸收性能, 降低电化学阻抗, 提高电化学活性比表面积(ECSA)。其中, BiZn2/Si最优的光电极电化学比表面积可达0.15 mF·cm-2。除此之外, 研究发现双金属共同作用有助于增强电极对中间体*OCHO的吸附作用, 在-0.8 V(vs. RHE)电势下, 最优的光电阴极BiZn2/Si生成HCOOH的法拉第效率高达96.1%。更重要的是, 光电阴极BiZn2/Si的光电流强度在10 h内维持-13 mA·cm-2, 表现出良好的性能稳定性。
中图分类号:
李成金, 薛怡, 周晓霞, 陈航榕. BiZnx/Si光电阴极的制备及其CO2还原性能研究[J]. 无机材料学报, 2022, 37(10): 1093-1101.
LI Chengjin, XUE Yi, ZHOU Xiaoxia, CHEN Hangrong. BiZnx/Si Photocathode: Preparation and CO2 Reduction Performance[J]. Journal of Inorganic Materials, 2022, 37(10): 1093-1101.
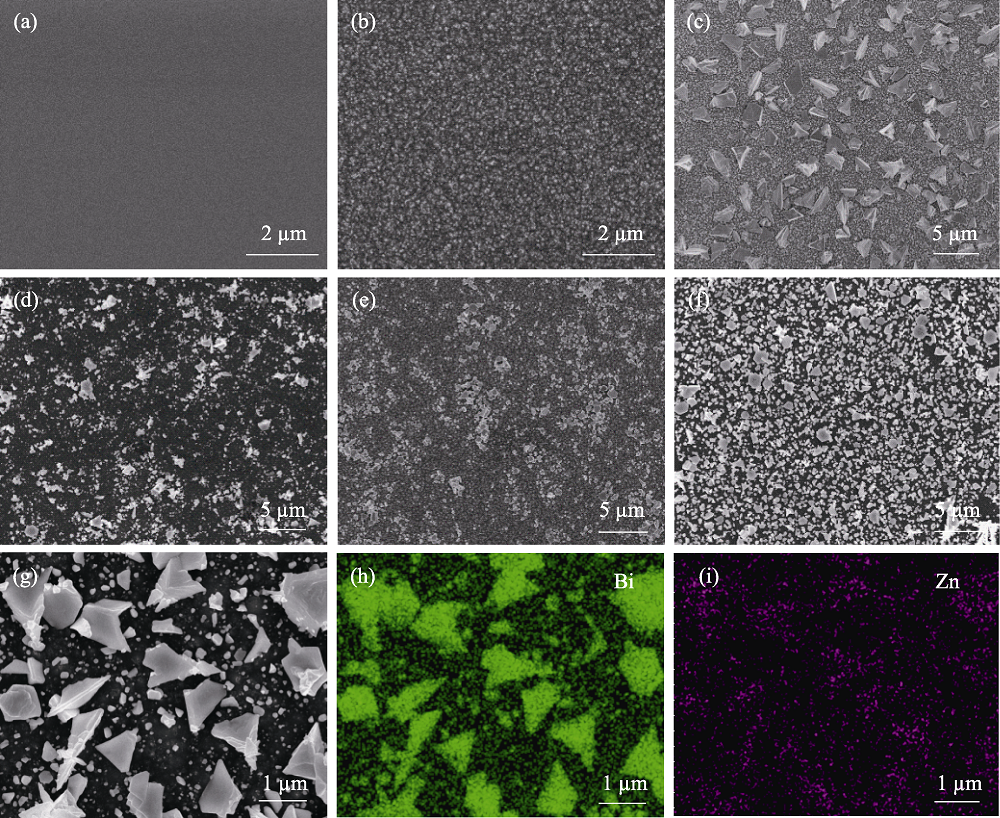
图3 (a) 未经处理的Si片、(b) 氢氧化钠碱溶液处理后的Si片、(c) Bi/Si、(d) BiZn1/Si、(e) BiZn2/Si和 (f) BiZn3/Si的SEM照片, (g~i) BiZn2/Si的元素分布图
Fig. 3 SEM images of (a) planar-Si, (b) Si treated with NaOH solution, (c) Bi/Si, (d) BiZn1/Si, (e) BiZn2/Si, and (f) BiZn3/Si with (g-i) elemental mappings of BiZn2/Si
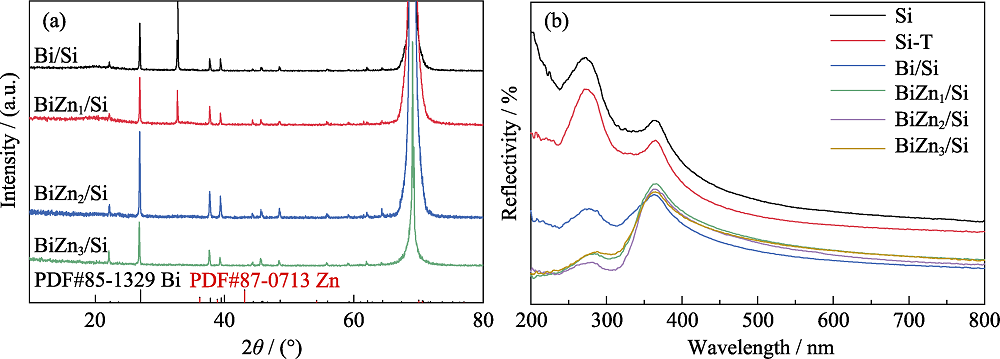
图4 (a) Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的 XRD图谱, (b) Planar-Si、Si-T、Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的紫外-可见反射谱图
Fig. 4 (a) XRD patterns of Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (b) UV-Vis reflectivity spectra of Planar-Si, Si-T, Bi/Si, BiZn1/Si, BiZn2/Si and BiZn3/Si Colorful figures are available on website
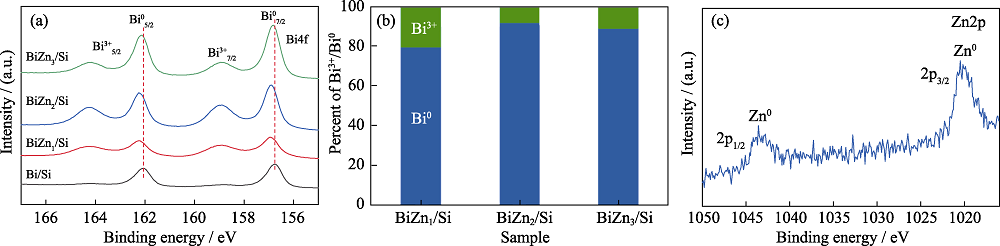
图5 (a) Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的Bi4f XPS精细谱图, (b) BiZn1/Si、BiZn2/Si和BiZn3/Si的Bi3+/Bi0比例, (c) BiZn2/Si的Zn2p XPS精细谱图
Fig. 5 (a) High resolution Bi4f XPS spectra of Bi/Si, BiZn1/Si, BiZn2/Si and BiZn3/Si, (b) ratios of Bi3+/Bi0 for BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (c) high resolution Zn2p XPS spectrum for BiZn2/Si Colorful figures are available on website
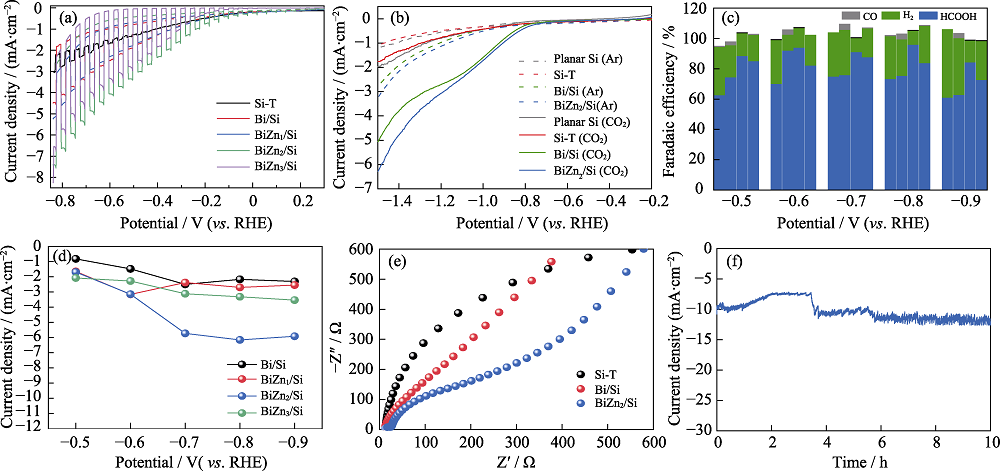
图6 (a) Si-T、Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的光电流响应曲线, (b)未经处理的Si片(Planar Si), Si-T、Bi/Si和BiZn2/Si在Ar和CO2饱和的0.5 mol·L-1 KHCO3溶液中的LSV曲线, Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的 (c) 还原产物的法拉第效率柱状图和(d) HCOOH分电流密度, (e) Si-T、Bi/Si和BiZn2/Si的电化学阻抗谱图, (f) BiZn2/Si的10 h稳定性测试图
Fig. 6 (a) Transient photocurrent curves of Si-T, Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, (b) LSV curves of untreated Si (Planar Si), Si-T, Bi/Si, and BiZn2/Si in Ar or CO2-saturated 0.5 mol·L-1 KHCO3 aqueous solution, (c) FE histograms of CO2 reduction productions and (d) HCOOH current densities for Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, (e) electrochemical impedance spectra (EIS) of Si-T, Bi/Si and BiZn2/Si, and (f) stability of BiZn2/Si during 10 h test Colorful figures are available on website
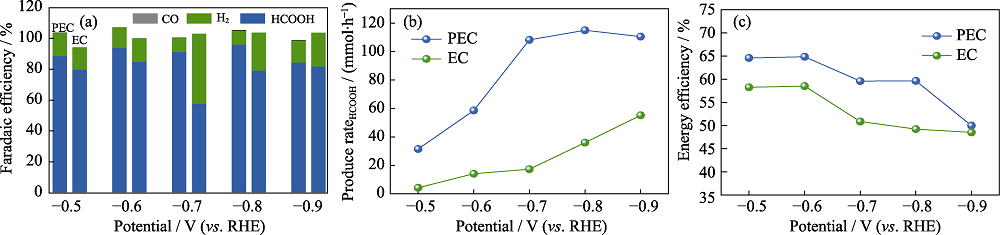
图7 样品BiZn2/Si的PEC和EC CO2还原性能对比
Fig. 7 PEC and EC CO2 reduction performances of BiZn2/Si (a) Faradaic efficiency of CO2 reduction products; (b) HCOOH production rates; (c) EE. Colorful figures are available on website
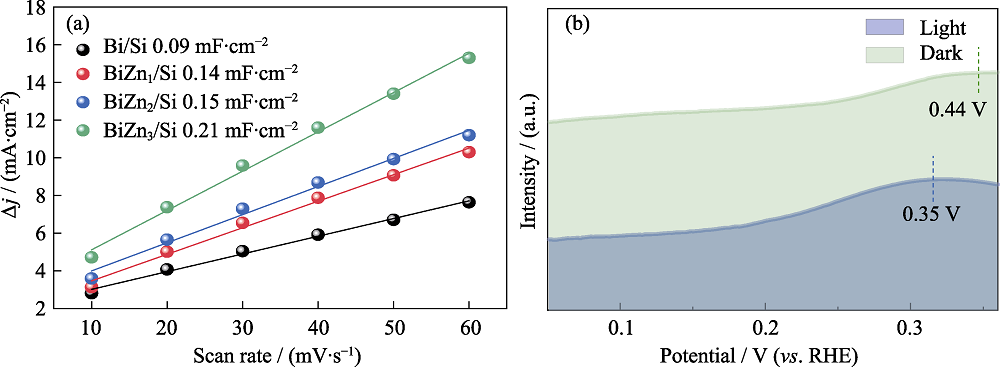
图8 (a) Bi/Si、BiZn1/Si、BiZn2/Si和BiZn3/Si的ECSA数据, (b) 在0.1 mol·L-1 KOH溶液中, BiZn2/Si的PEC和EC CO2还原的氧化LSV曲线
Fig. 8 (a) ECSA lines of Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (b) oxidation LSV curves of PEC and EC CO2 reduction for BiZn2/Si in 0.1 mol·L-1 KOH aqueous solution
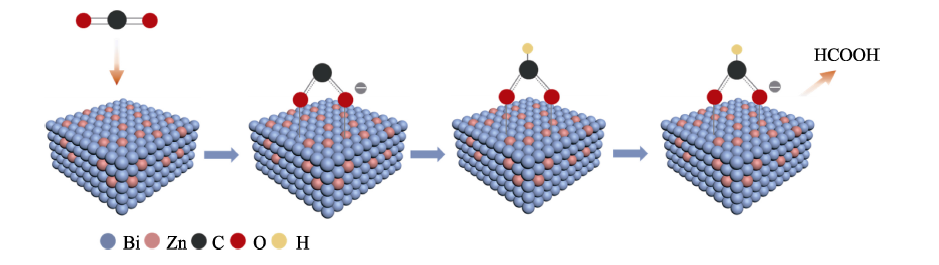
图9 CO2在光电阴极BiZn2/Si上形成HCOOH的机理示意图
Fig. 9 Mechanismic schematic of formation process of HCOOH on the photocathode BiZn2/Si Colorful figure is available on website
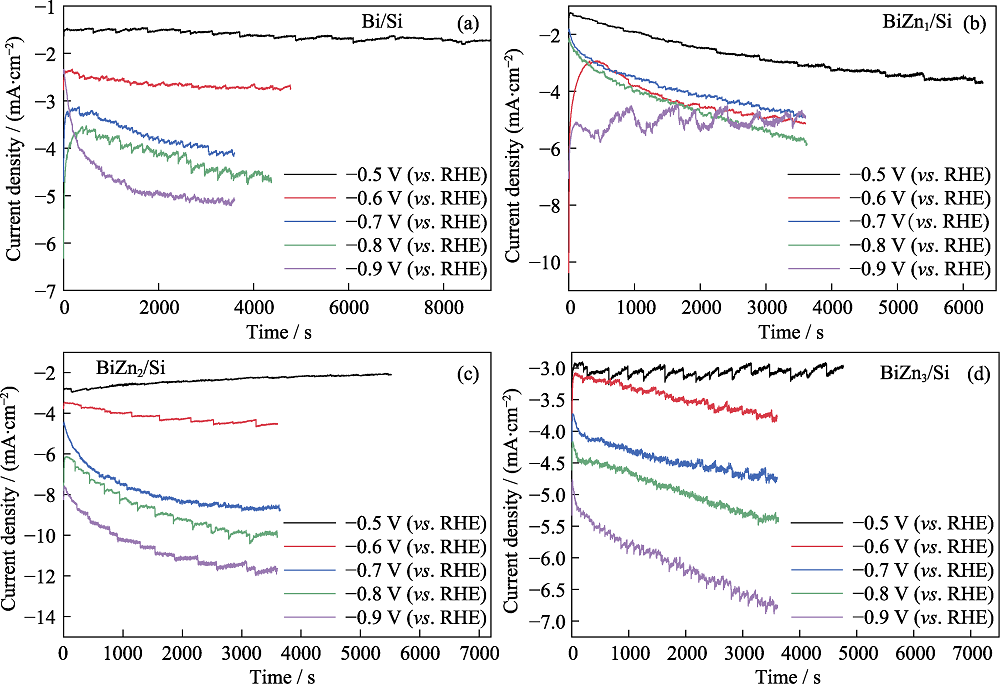
图S3 (a) Bi/Si、(b) BiZn1/Si、(c) BiZn2/Si和(d) BiZn3/Si的电流-时间曲线
Fig. S3 i-t curves of (a) Bi/Si, (b) BiZn1/Si, (c) BiZn2/Si and (d) BiZn3/Si Colorful figures are available on website
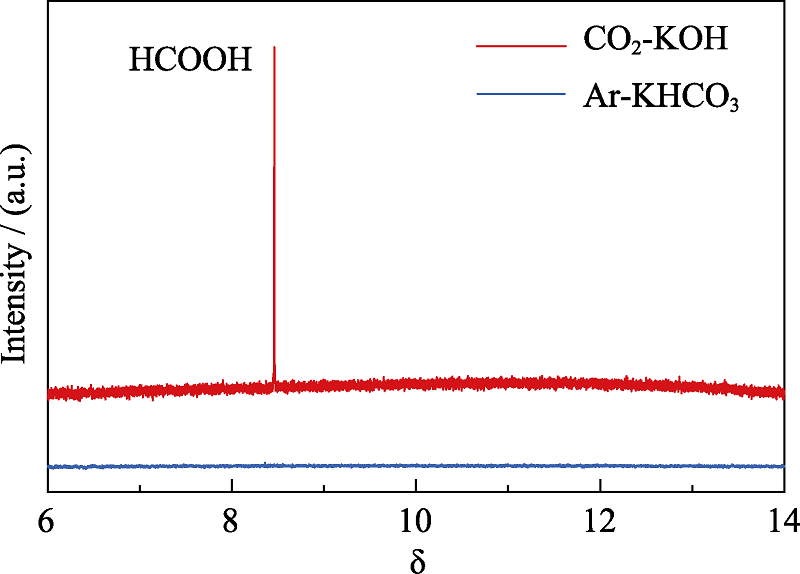
图S6 BiZn2/Si在CO2饱和的0.5 mol·L-1 KOH和Ar饱和的0.5 mol·L-1 KHCO3溶液中光电还原CO2反应后液相产物的1H NMR图谱
Fig. S6 1H NMR spectrum of the liquid phase products of BiZn2/Si in CO2-saturated 0.5 mol·L-1 KOH and Ar-saturated 0.5 mol·L-1 KHCO3 solutions for photoelechemical reduction of CO2
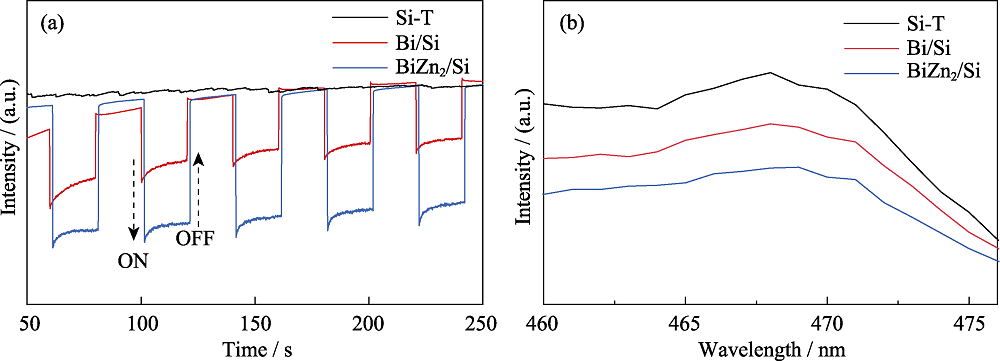
图S7 (a) BiZn2/Si、Bi/Si 和Si-T的光电流响应曲线, (b) BiZn2/Si、Bi/Si 和Si-T的荧光光谱图
Fig. S7 (a) Photocurrent curves of BiZn2/Si, Bi/Si and Si-T, (b) PL spectra of BiZn2/Si, Bi/Si and Si-T
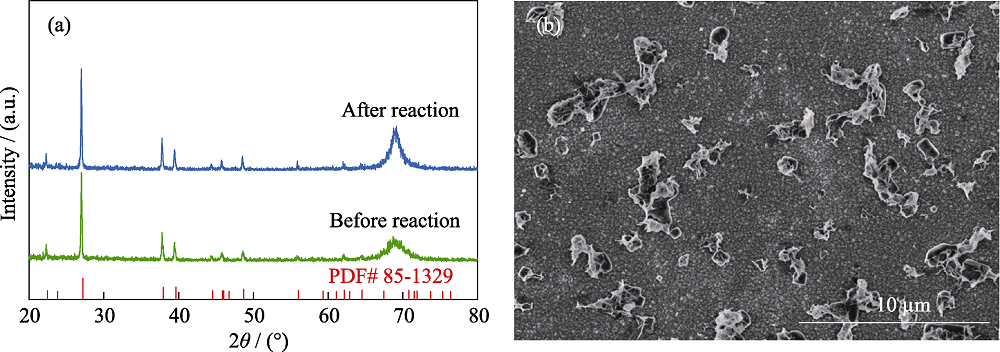
图S9 BiZn2/Si稳定性测试前后的 (a) XRD图谱和 (b) 稳定性测试后的SEM照片
Fig. S9 (a) XRD patterns of BiZn2/Si before and after stability test, (b) typical SEM image of BiZn2/Si after stability test
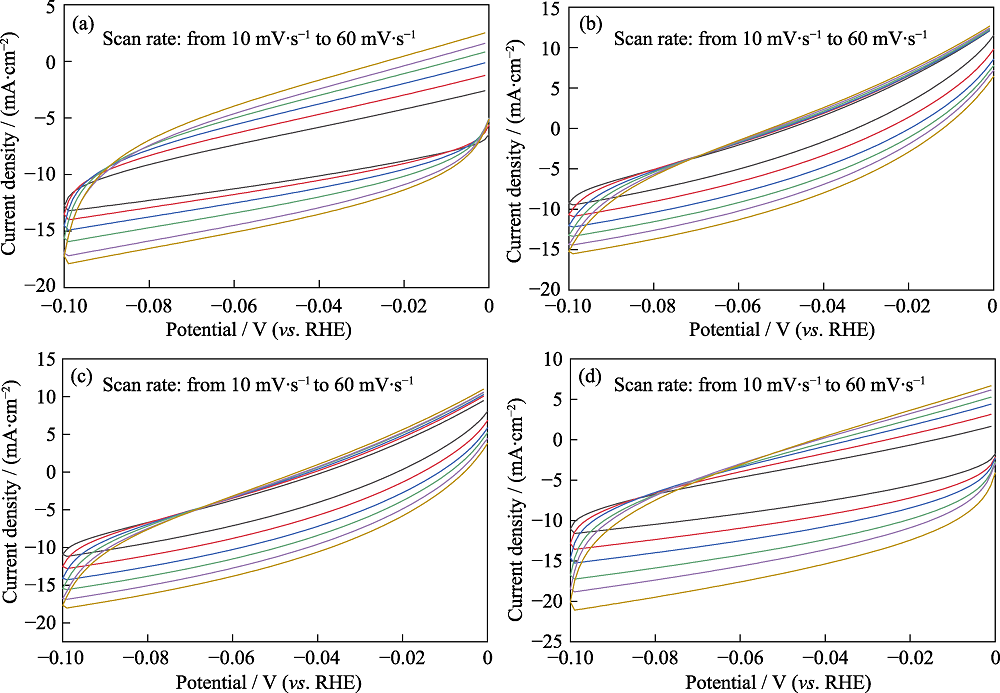
图S10 (a) Bi/Si、(b) BiZn1/Si、(c) BiZn2/Si和 (d) BiZn3/Si在不同扫描速率下的CV曲线
Fig. S10 CV curves for (a) Bi/Si, (b) BiZn1/Si, (c) BiZn2/Si and (d) BiZn3/Si at different scan rates
| Sample | BiZn1/Si | BiZn2/Si | BiZn3/Si |
|---|---|---|---|
| (n)Bi/(n)Zn | 142 | 120 | 88 |
表S1 ICP结果中不同样品的Bi/Zn摩尔比例
Table S1 Bi/Zn molar ratios of different samples in ICP results
| Sample | BiZn1/Si | BiZn2/Si | BiZn3/Si |
|---|---|---|---|
| (n)Bi/(n)Zn | 142 | 120 | 88 |
| Electrode | Electrolyte | Eapp/V | Jtotal/(mA·cm-2) | FEformate | Ref. |
|---|---|---|---|---|---|
| Sn/SnOx | 0.5 mol·L-1 KHCO3 | -0.7 vs. RHE | -2 | ~38% PEC | [ |
| SnO2 | 0.5 mol·L-1 NaOH | -0.6 vs. RHE | -3.5 | 67.6% EC | [ |
| Sn foil | 0.5 mol·L-1 KHCO3 | -2.0 vs. SCE | -28 | 63.5% EC | [ |
| Sn dendrite | 0.1 mol·L-1 KHCO3 | -1.36 vs. RHE | -17.1 | 71.6% EC | [ |
| Sn GDE | 0.5 mol·L-1 KHCO3 | -1. 8 vs. SCE | -22.2 | 78.6% EC | [ |
| 2,2’-bpy-coordinated Cu | 0.5 mol·L-1 KHCO3 | -1.2 vs. RHE | -15 | 57.7% PEC | [ |
| Si/Bi5 | 0.5 mol·L-1 KHCO3 | -1.03 vs. RHE | -24.1 | 72.1% PEC | [ |
| Bi-PMo nanosheets | 0.5 mol·L-1 NaHCO3 | -0.86 vs. RHE | -30 | 93% EC | [ |
| Bi2O3 nanoparticle | 0.5 mol·L-1 NaHCO3 | -1.2 vs. RHE | -22 | 91% EC | [ |
| p-Si/Bi | 0.5 mol·L-1 NaHCO3 | -0.9 vs. RHE | -12 | 90% PEC | [ |
| Bi nanoflakes | 0.1 mol·L-1 KHCO3 | -0.4 vs. RHE | - | 79.5% EC | [ |
| Cu25In75 | 0.5 mol·L-1 NaHCO3 | -0.7 vs. RHE | - | 84.1% EC | [ |
| In1.5Cu0.5 NPs | 0.1 mol·L-1 KHCO3 | -1.2 vs. RHE | -3.59 | 90% EC | [ |
| This work | 0.5 mol·L-1 KHCO3 | -0.8 vs. RHE | -6.45 | 96.1% PEC | This work |
表S2 不同光/电催化剂的法拉第效率和电流密度对比
Table S2 Faraday efficiency and current density comparison of different photo/electrocatalysts
| Electrode | Electrolyte | Eapp/V | Jtotal/(mA·cm-2) | FEformate | Ref. |
|---|---|---|---|---|---|
| Sn/SnOx | 0.5 mol·L-1 KHCO3 | -0.7 vs. RHE | -2 | ~38% PEC | [ |
| SnO2 | 0.5 mol·L-1 NaOH | -0.6 vs. RHE | -3.5 | 67.6% EC | [ |
| Sn foil | 0.5 mol·L-1 KHCO3 | -2.0 vs. SCE | -28 | 63.5% EC | [ |
| Sn dendrite | 0.1 mol·L-1 KHCO3 | -1.36 vs. RHE | -17.1 | 71.6% EC | [ |
| Sn GDE | 0.5 mol·L-1 KHCO3 | -1. 8 vs. SCE | -22.2 | 78.6% EC | [ |
| 2,2’-bpy-coordinated Cu | 0.5 mol·L-1 KHCO3 | -1.2 vs. RHE | -15 | 57.7% PEC | [ |
| Si/Bi5 | 0.5 mol·L-1 KHCO3 | -1.03 vs. RHE | -24.1 | 72.1% PEC | [ |
| Bi-PMo nanosheets | 0.5 mol·L-1 NaHCO3 | -0.86 vs. RHE | -30 | 93% EC | [ |
| Bi2O3 nanoparticle | 0.5 mol·L-1 NaHCO3 | -1.2 vs. RHE | -22 | 91% EC | [ |
| p-Si/Bi | 0.5 mol·L-1 NaHCO3 | -0.9 vs. RHE | -12 | 90% PEC | [ |
| Bi nanoflakes | 0.1 mol·L-1 KHCO3 | -0.4 vs. RHE | - | 79.5% EC | [ |
| Cu25In75 | 0.5 mol·L-1 NaHCO3 | -0.7 vs. RHE | - | 84.1% EC | [ |
| In1.5Cu0.5 NPs | 0.1 mol·L-1 KHCO3 | -1.2 vs. RHE | -3.59 | 90% EC | [ |
| This work | 0.5 mol·L-1 KHCO3 | -0.8 vs. RHE | -6.45 | 96.1% PEC | This work |
| [1] |
ZHU D, LIU J, QIAO S. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Advanced Materials, 2016, 28(18): 3423-3452.
DOI URL |
| [2] |
COSTENTIN C, ROBERT M, SAVEANT J. Catalysis of the electrochemical reduction of carbon dioxide. Chemical Society Reviews, 2013, 42(6): 2423-2436.
DOI PMID |
| [3] |
QIAO J, LIU Y, HONG F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chemical Society Reviews, 2014, 43(2): 631-675.
DOI PMID |
| [4] | LIANG Z, SHEN R, NG Y, et al. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. Journal of Materials Science & Technology, 2020, 56: 89-121. |
| [5] |
LI X, WEN J, LOW J, et al. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Science China Materials, 2014, 57: 70-100.
DOI URL |
| [6] |
SHEN R, REN D, DING Y, et al. Nanostructured CdS for efficient photocatalytic H2 evolution: a review. Science China Materials, 2020, 63(11): 2153-2188.
DOI URL |
| [7] |
LI X, YU J, JARONIEC M, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chemical Reviews, 2019, 119(6): 3962-4179.
DOI URL |
| [8] |
JOUNY M, LUC W, JIAO F. General techno-economic analysis of CO2 electrolysis systems. Industrial & Engineering Chemistry Research, 2018, 57(6): 2165-2177.
DOI URL |
| [9] |
SPONHOLZ P, MELLMANN D, JUNGE H, et al. Towards a practical setup for hydrogen production from formic acid. ChemSusChem, 2013, 6(7): 1172-1176.
DOI PMID |
| [10] |
AGARWAL A, ZHAI Y, HILL D, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility. ChemSusChem, 2011, 4(9): 1301-1310.
DOI PMID |
| [11] |
HORI Y, WAKEBE H, TSUKAMOTO T, et al. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochimica Acta, 1994, 39(11): 1833-1839.
DOI URL |
| [12] |
KIM S, DONG W, GIM S, et al. Shape-controlled bismuth nanoflakes as highly selective catalysts for electrochemical carbon dioxide reduction to formate. Nano Energy, 2017, 39: 44-52.
DOI URL |
| [13] |
HAN N, WANG Y, YANG H, et al. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nature Communications, 2018, 9(1): 1320.
DOI URL |
| [14] |
HE S, NI F, WANG L, et al. The p-orbital delocalization of main-group metals to boost CO2 electroreduction. Angewandte Chemie International Edition, 2018, 57(49): 16114-16119.
DOI URL |
| [15] |
MIAO C, YUAN G. Morphology-controlled Bi2O3 nanoparticles as catalysts for selective electrochemical reduction of CO2 to formate. ChemElectroChem, 2018, 5(23): 3741-3747.
DOI URL |
| [16] |
YANG H, HAN N, DENG J, et al. Selective CO2 reduction on 2D mesoporous Bi nanosheets. Advanced Energy Materials, 2018, 8(35): 1801536.
DOI URL |
| [17] |
GUO S, ZHANG Y, ZHANG X, et al. Phosphomolybdic acid-assisted growth of ultrathin bismuth nanosheets for enhanced electrocatalytic reduction of CO2 to formate. ChemSusChem, 2019, 12(5): 1091-1100.
DOI URL |
| [18] |
LU P, GAO D, HE H, et al. Facile synthesis of a bismuth nanostructure with enhanced selectivity for electrochemical conversion of CO2 to formate. Nanoscale, 2019, 11(16): 7805-7812.
DOI URL |
| [19] |
BHUPENDRA K, MARK L, JESSE F, et al. Photochemical and photoelectrochemical reduction of CO2. The Annual Review of Physical Chemistry, 2012, 63(1): 541-569.
DOI URL |
| [20] |
SUN K, SHEN S, LIANG Y, et al. Enabling silicon for solar-fuel production. Chemical Reviews, 2014, 114(17): 8662-8719.
DOI PMID |
| [21] |
WHITE J, BARUCH M, PANDER J, et al. Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chemical Reviews, 2015, 115(23): 12888-12935.
DOI PMID |
| [22] |
MAEDA K. Metal-complex/semiconductor hybrid photocatalysts and photoelectrodes for CO2 reduction driven by visible light. Advanced Materials, 2019, 31(25): 1808205.
DOI URL |
| [23] |
DING P, HU Y, DENG J, et al. Controlled chemical etching leads to efficient silicon-bismuth interface for photoelectrochemical CO2 reduction to formate. Materials Today Chemistry, 2019, 11: 80-85.
DOI URL |
| [24] |
GONG Q, DING P, XU M, et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nature Communications, 2019, 10(1): 2807.
DOI PMID |
| [25] |
LI Z, FENG Y, LI Y, et al. Fabrication of Bi/Sn bimetallic electrode for high-performance electrochemical reduction of carbon dioxide to formate. Chemical Engineering Journal, 2022, 428: 130901.
DOI URL |
| [26] |
EON H, TIMOSHENKO J, SCHOLTEN F, et al. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. Journal of the American Chemical Society, 2019, 141(50): 19879-19887.
DOI URL |
| [27] |
KUMAR A, BUI V, LEE J, et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nature Communications, 2021, 12(1): 6766.
DOI PMID |
| [28] |
WU Z, CARACCIOLO D, MASWADEH Y, et al. Alloying-realloying enabled high durability for Pt-Pd-3D-transition metal nanoparticle fuel cell catalysts. Nature Communications, 2021, 12(1): 859.
DOI URL |
| [29] |
LEE W, KO Y, KIM J, et al. High crystallinity design of Ir-based catalysts drives catalytic reversibility for water electrolysis and fuel cells. Nature Communications, 2021, 12(1): 4271.
DOI PMID |
| [30] |
WANG Y, LI Y, LIU J, et al. BiPO4-derived 2D nanosheets for efficient electrocatalytic reduction of CO2 to liquid fuel. Angewandte Chemie International Edition, 2021, 60(14): 7681-7685.
DOI URL |
| [31] |
LUO W, ZHANG J, LI M, et al. Boosting CO production in electrocatalytic CO2 reduction on highly porous Zn catalysts. ACS Catalysis, 2019, 9(5): 3783-3791.
DOI URL |
| [32] |
ZHANG X, SU X, ZHENG Y, et al. Strongly coupled cobalt diselenide monolayers for selective electrocatalytic oxygen reduction to H2O2 under acidic conditions. Angewandte Chemie International Edition, 2019, 60: 26922-26931.
DOI URL |
| [1] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [2] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [3] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [4] | 田建建, 马霞, 王敏, 姚鹤良, 华子乐, 张玲霞. 锡量子点制备及其电催化还原二氧化碳产甲酸性能[J]. 无机材料学报, 2021, 36(12): 1337-1342. |
| [5] | 张清明, 朱敏, 周晓霞. CuO/ZnO复合电催化剂的制备及其还原CO2制合成气[J]. 无机材料学报, 2021, 36(11): 1145-1153. |
| [6] | 刘亚鑫, 王敏, 沈梦, 王强, 张玲霞. 铋掺杂提高氧化铈中氧空位浓度增强CO2光催化还原性能[J]. 无机材料学报, 2021, 36(1): 88-94. |
| [7] | 孙海健,刘惠玲. 铈掺杂TiO2/Ti光电极制备及可见光下光电催化性能的研究[J]. 无机材料学报, 2007, 22(6): 1065-1069. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||