无机材料学报 ›› 2021, Vol. 36 ›› Issue (7): 766-772.DOI: 10.15541/jim20200498 CSTR: 32189.14.10.15541/jim20200498
所属专题: 【虚拟专辑】超级电容器(2020~2021); 【能源环境】超级电容器(202409)
孙鹏1,2( ), 张绍宁1,3, 毕辉1, 董武杰1, 黄富强1,3,4(
), 张绍宁1,3, 毕辉1, 董武杰1, 黄富强1,3,4( )
)
收稿日期:2020-08-27
修回日期:2020-10-20
出版日期:2021-07-20
网络出版日期:2020-11-05
通讯作者:
黄富强, 研究员. E-mail:huangfq@mail.sic.ac.cn
作者简介:孙鹏(1992-), 男, 博士研究生. E-mail:sunpeng@student.sic.ac.cn
SUN Peng1,2( ), ZHANG Shaoning1,3, BI Hui1, DONG Wujie1, HUANG Fuqiang1,3,4(
), ZHANG Shaoning1,3, BI Hui1, DONG Wujie1, HUANG Fuqiang1,3,4( )
)
Received:2020-08-27
Revised:2020-10-20
Published:2021-07-20
Online:2020-11-05
Contact:
HUANG Fuqiang, professor. E-mail:huangfq@mail.sic.ac.cn
About author:SUN Peng (1992-), male, PhD candidate. E-mail:sunpeng@student.sic.ac.cn
Supported by:摘要:
碳材料是极具潜力的超级电容器电极材料, 但是其容量较低。异质原子掺杂, 尤其是氮掺杂, 是大幅度提高碳材料电化学性能的有效方法。但是在碳材料中实现高含量的活性氮掺杂仍极具挑战。本研究通过Si-O-Si网络和氧化铝之间的相互作用成功调节碳材料的掺氮种类及其含量。除此之外, 通过调节前驱体组成, 碳材料的结构可以从珊瑚状转变为三维结构。在反应中, 氧化物中的氧原子可以和碳材料中氮原子成键, 氮原子不易逃离, 从而实现高含量氮掺杂(5.29at%@1000 ℃)。另一方面, 相互作用使碳材料孔体积增大(1.78 m3·g-1)和孔径分布加宽(0.5~60 nm)。因此, 获得的富氮掺杂碳材料具有302 F·g-1@1 A·g-1的高容量和177 Fg-1@120 A·g-1的杰出倍率性能。此独特的固氮方法是一种有潜力的制备高性能超级电容器电极材料的策略。
中图分类号:
孙鹏, 张绍宁, 毕辉, 董武杰, 黄富强. 基于结构调节碳材料的掺氮种类和含量及其超级电容器储能应用[J]. 无机材料学报, 2021, 36(7): 766-772.
SUN Peng, ZHANG Shaoning, BI Hui, DONG Wujie, HUANG Fuqiang. Tuning Nitrogen Species and Content in Carbon Materials through Constructing Variable Structures for Supercapacitors[J]. Journal of Inorganic Materials, 2021, 36(7): 766-772.
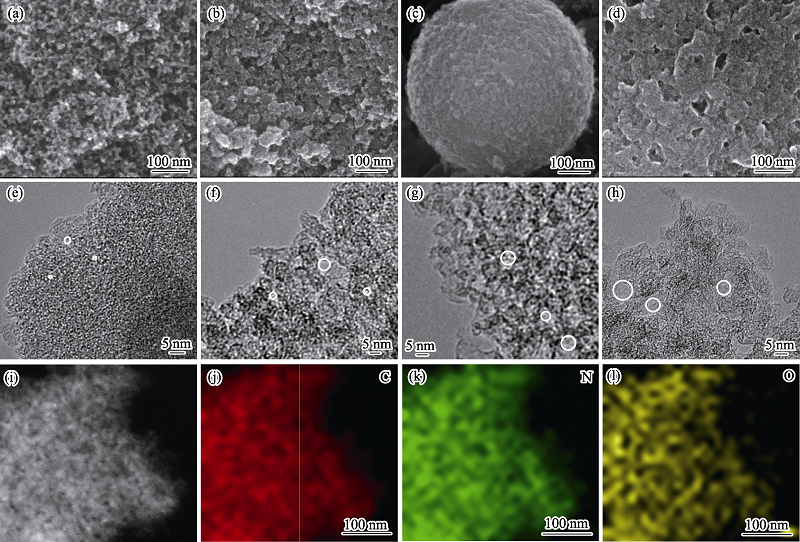
Fig. 2 (a-d) SEM and (e-h) HRTEM images of (a, e) NC, (b, f) SiO-NC, (c, g) AlO-NC and (d, h) SiAlO-NC, and (i-l) energy dispersive spectroscopy (EDS) elemental mappings of SiAlO-NC
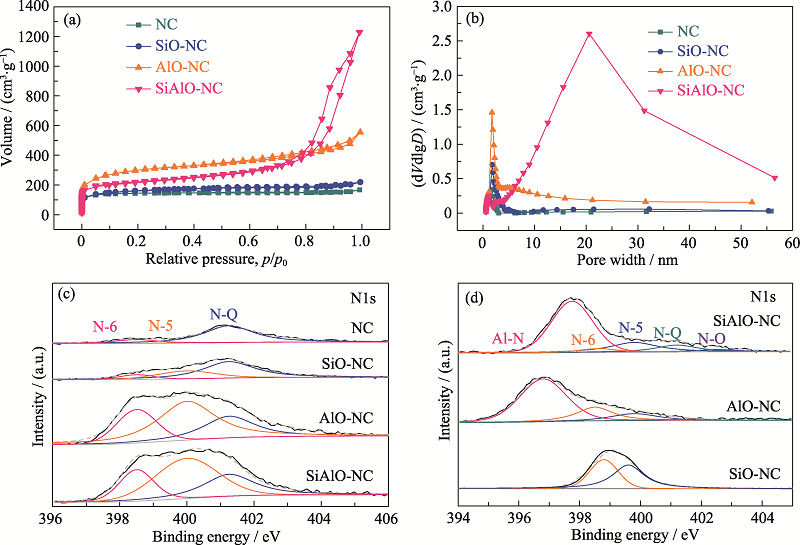
Fig. 3 (a) Nitrogen adsorption-desorption isotherms and (b) pore size distributions of NC, SiO-NC, AlO-NC, SiAlO-NC (after removing templates), N1s XPS spectra of (c) NC, SiO-NC, AlO-NC, SiAlO-NC (after removing templates) and (d) SiO-NC, AlO-NC, SiAlO-NC (without removing templates)
| Sample | SSA /(m2·g-1) | Pores volume /(cm3·g-1) | N /at% | N-6 /at% | N-5 /at% |
|---|---|---|---|---|---|
| NC | 440.78 | 0.07 | 1.42 | 0.21 | 0.03 |
| SiO-NC | 520.78 | 0.19 | 1.66 | 0.14 | 0.44 |
| AlO-NC | 980.35 | 0.68 | 3.52 | 0.78 | 1.75 |
| SiAlO-NC | 703.20 | 1.78 | 5.29 | 1.17 | 2.53 |
Table 1 BET and elemental parameters of samples
| Sample | SSA /(m2·g-1) | Pores volume /(cm3·g-1) | N /at% | N-6 /at% | N-5 /at% |
|---|---|---|---|---|---|
| NC | 440.78 | 0.07 | 1.42 | 0.21 | 0.03 |
| SiO-NC | 520.78 | 0.19 | 1.66 | 0.14 | 0.44 |
| AlO-NC | 980.35 | 0.68 | 3.52 | 0.78 | 1.75 |
| SiAlO-NC | 703.20 | 1.78 | 5.29 | 1.17 | 2.53 |
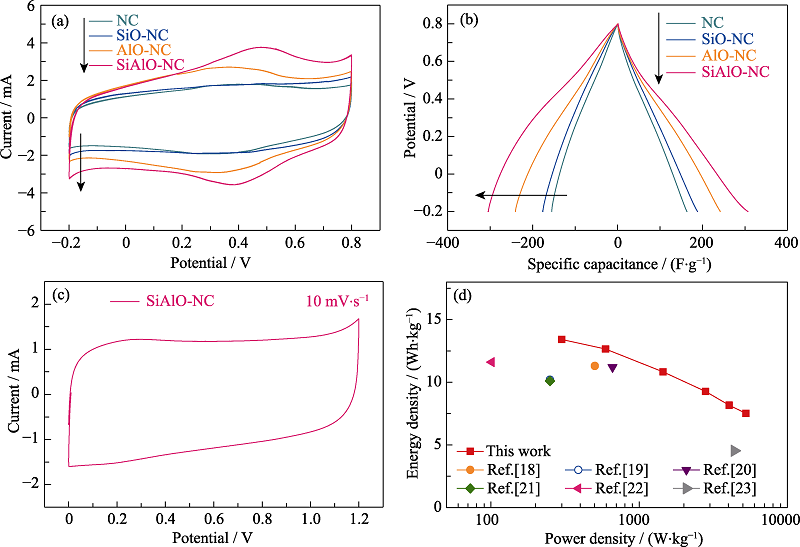
Fig. 4 (a) CV curves at 10 mV·s-1 and (b) galvanostatic charge/discharge (GCD) curves of NC, SiO-NC, AlO-NC, SiAlO-NC at 1 A·g-1 in three-electrode configuration; (c) CV curve of symmetric cell with SiAlO-NC, and (d) Ragone plots for SiAlO-NC and other nitrogen-carbon materials
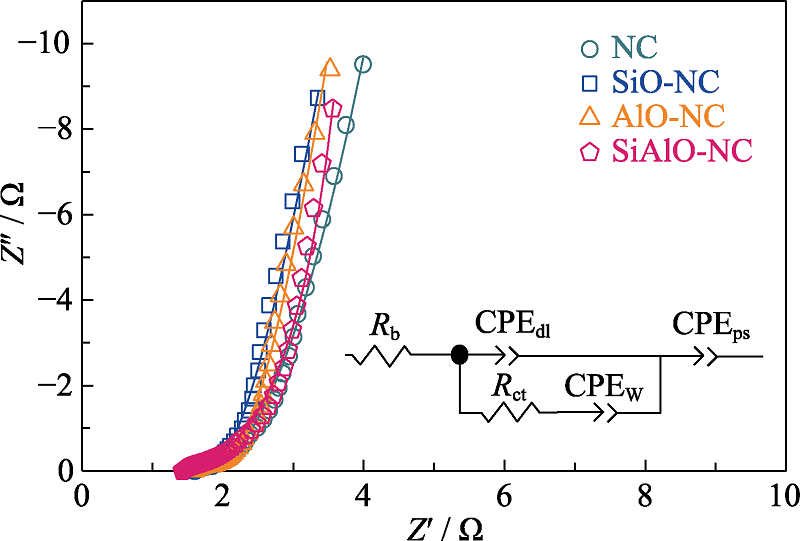
Fig. S10 Nyquist plots over 0.01 to 105 Hz of NC, SiO-NC, AlO-NC and SiAlO-NC based on the fittings using equivalent Randles circuit model(inset) in three-electrode configuration
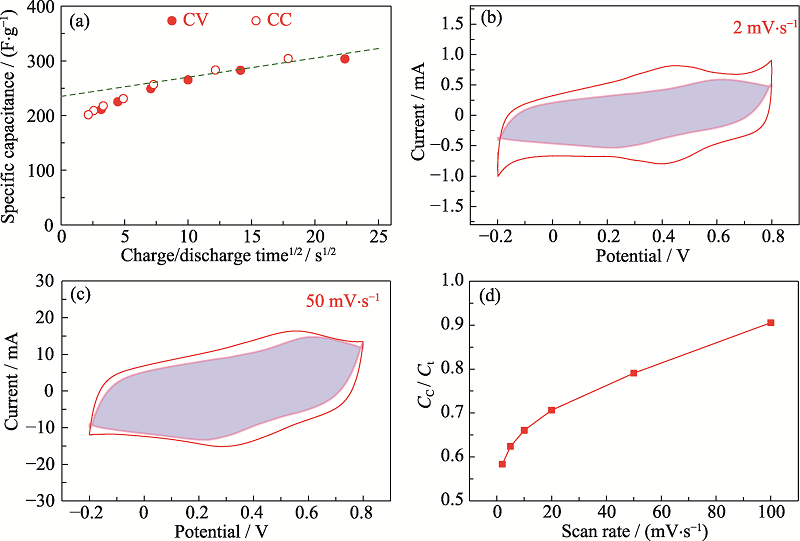
Fig. S11 Electrochemical performance of the SiAlO-NC sample(a) Capacitance versus discharge time, t1/2. Hollow symbols: CC test data, solid symbols: CV test data from 2 to 100 mV·s-1; Extrapolation of capacitance to t=0 gives a rate-independent capacitance; Instantaneous current of the SiAlO-NC sample at (b) 2 and (c) 50 mV·s-1, giving the shaded loop is the capacitive capacitance, and the region outside is the pseudocapacitance; (d) Fraction of capacitive capacitance Cc in total capacitance Ct
| Sample | N/at% | N-6/at% | N-5/at% | N-Q/at% | Sample | N/at% | N-6/at% | N-5/at% | N-Q/at% |
|---|---|---|---|---|---|---|---|---|---|
| NC (900 ℃) | 2.81 | 1.54 | 0.82 | 0.45 | NC (1100 ℃) | 0.61 | 0.03 | 0.32 | 0.26 |
| SiAlO-NC (900 ℃) | 8.27 | 3.65 | 3.00 | 1.62 | SiAlO-NC (1100 ℃) | 4.33 | 1.61 | 1.27 | 1.45 |
Table S1 Characteristic summary of NC and SiAlO-NC
| Sample | N/at% | N-6/at% | N-5/at% | N-Q/at% | Sample | N/at% | N-6/at% | N-5/at% | N-Q/at% |
|---|---|---|---|---|---|---|---|---|---|
| NC (900 ℃) | 2.81 | 1.54 | 0.82 | 0.45 | NC (1100 ℃) | 0.61 | 0.03 | 0.32 | 0.26 |
| SiAlO-NC (900 ℃) | 8.27 | 3.65 | 3.00 | 1.62 | SiAlO-NC (1100 ℃) | 4.33 | 1.61 | 1.27 | 1.45 |
| Carbon material | Specific capacitance /(F·g-1) | Rate capability /(F·g-1) | Cycling performance | Ref. |
|---|---|---|---|---|
| N-doped porous carbon | 327 at 1 A·g-1 | 200 at 20 A·g-1 | 10000 cycles@100% | [2] |
| N/S co-doped porous carbon | 272 at 1 A·g-1 | 172 at 100 A·g-1 | 5000 cycles at 5 A·g-1@97.1% | [3] |
| N/O co-doped carbon | 242 at 0.5 A·g-1 | 132 at 20 A·g-1 | 10000 cycles at 5 A·g-1@97% | [4] |
| Graphene/N-rich carbon | 229 at 1 A·g-1 | 196 at 10 A·g-1 | 10000 cycles at 2 A·g-1@99.5% | [5] |
| N-doped carbon foam | 280 at 1 A·g-1 | 185 at 40 A·g-1 | 10000 cycles at 5 A·g-1@96.3% | [6] |
| N-doped tubular carbon | 204 at 0.1 A·g-1 | 173 at 10 A·g-1 | 50000 cycles at 5 A·g-1@91.5% | [7] |
| N-doped carbon microtube | 309 at 1 A·g-1 | 220 at 10 A·g-1 | 10000 cycles at 1 A·g-1@94% | [8] |
| N-doped porous carbon | 292 at 1 A·g-1 | 200 at 20 A·g-1 | 10000 cycles at 1 A·g-1@86% | [9] |
| N-doped carbon nanorod | 271 at 0.5 A·g-1 | 175 at 20 A·g-1 | 10000 cycles at 5 A·g-1@97% | [10] |
| 3D porous carbon | 261 at 0.5 A·g-1 | 200 at 10 A·g-1 | 5000 cycles at 1 A·g-1@96% | [11] |
| N-doped porous carbon | 250 at 1.0 A·g-1 | 160 at 10 A·g-1 | 3000 cycles at 1 A·g-1@97.3% | [12] |
| N-doped carbon spheres | 301 at 0.2 A·g-1 | 210 at 5 A·g-1 | 5000 cycles at 5 A·g-1@100% | [13] |
| N-doped porous carbon | 252 at 1.0 A·g-1 | 189 at 15 A·g-1 | 10000 cycles at 15 A·g-1@94% | [14] |
| N-doped porous carbon | 334 at 1.0 A·g-1 | 215 at 20 A·g-1 | 10000 cycles at 20 mV·s-1@95.2% | [15] |
| 3D graphene-like carbon | 252 at 1.0 A·g-1 | 168 at 50 A·g-1 | 5000 cycles at 50 mV·s-1@98% | [16] |
| SiAlO-NC | 302 at 1 A·g-1 | 218 at 20 A·g-1 | 20000 cycles at 20 mV·s-1@92% | This work |
| 177 at 120 A·g-1 |
Table S2 Comparison of the specific capacitances, rate capabilities and cycling performances for previously reported N-doped porous carbon materials
| Carbon material | Specific capacitance /(F·g-1) | Rate capability /(F·g-1) | Cycling performance | Ref. |
|---|---|---|---|---|
| N-doped porous carbon | 327 at 1 A·g-1 | 200 at 20 A·g-1 | 10000 cycles@100% | [2] |
| N/S co-doped porous carbon | 272 at 1 A·g-1 | 172 at 100 A·g-1 | 5000 cycles at 5 A·g-1@97.1% | [3] |
| N/O co-doped carbon | 242 at 0.5 A·g-1 | 132 at 20 A·g-1 | 10000 cycles at 5 A·g-1@97% | [4] |
| Graphene/N-rich carbon | 229 at 1 A·g-1 | 196 at 10 A·g-1 | 10000 cycles at 2 A·g-1@99.5% | [5] |
| N-doped carbon foam | 280 at 1 A·g-1 | 185 at 40 A·g-1 | 10000 cycles at 5 A·g-1@96.3% | [6] |
| N-doped tubular carbon | 204 at 0.1 A·g-1 | 173 at 10 A·g-1 | 50000 cycles at 5 A·g-1@91.5% | [7] |
| N-doped carbon microtube | 309 at 1 A·g-1 | 220 at 10 A·g-1 | 10000 cycles at 1 A·g-1@94% | [8] |
| N-doped porous carbon | 292 at 1 A·g-1 | 200 at 20 A·g-1 | 10000 cycles at 1 A·g-1@86% | [9] |
| N-doped carbon nanorod | 271 at 0.5 A·g-1 | 175 at 20 A·g-1 | 10000 cycles at 5 A·g-1@97% | [10] |
| 3D porous carbon | 261 at 0.5 A·g-1 | 200 at 10 A·g-1 | 5000 cycles at 1 A·g-1@96% | [11] |
| N-doped porous carbon | 250 at 1.0 A·g-1 | 160 at 10 A·g-1 | 3000 cycles at 1 A·g-1@97.3% | [12] |
| N-doped carbon spheres | 301 at 0.2 A·g-1 | 210 at 5 A·g-1 | 5000 cycles at 5 A·g-1@100% | [13] |
| N-doped porous carbon | 252 at 1.0 A·g-1 | 189 at 15 A·g-1 | 10000 cycles at 15 A·g-1@94% | [14] |
| N-doped porous carbon | 334 at 1.0 A·g-1 | 215 at 20 A·g-1 | 10000 cycles at 20 mV·s-1@95.2% | [15] |
| 3D graphene-like carbon | 252 at 1.0 A·g-1 | 168 at 50 A·g-1 | 5000 cycles at 50 mV·s-1@98% | [16] |
| SiAlO-NC | 302 at 1 A·g-1 | 218 at 20 A·g-1 | 20000 cycles at 20 mV·s-1@92% | This work |
| 177 at 120 A·g-1 |
| [1] |
ZHANG L L, ZHAO X S. Carbon-based materials as supercapacitor electrodes. Chemical Society Reviews, 2009,38:2520-2531.
DOI URL |
| [2] |
HAO Y X, QIAN M, XU J J, et al. Porous cotton-derived carbon: synthesis, microstructure and supercapacitive performance. Journal of Inorganic Materials, 2018,33(1):93-99.
DOI URL |
| [3] |
YU J H, XU L L, ZHU Q Q, et al. Superior electrochemical performance of graphene via carboxyl functionalization and surfactant intercalation. Journal of Inorganic Materials, 2016,31(2):220-224.
DOI URL |
| [4] |
HWANG J Y, LI M P, EI-KADY M F, et al. Next-generation activated carbon supercapacitors: a simple step in electrode processing leads to remarkable gains in energy density. Advanced Functional Materials, 2017,27:1605745.
DOI URL |
| [5] |
ZHOU Y, JIA Z X, SHI L L, et al. Pressure difference-induced synthesis of P-doped carbon nanobowls for high-performance supercapacitors. Chemical Engineering Journal, 2020,385:123858.
DOI URL |
| [6] |
SEREDYCH M, HULICOVA-JURCAKOVA D, LU G Q, et al. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon, 2008,46:1475-1488.
DOI URL |
| [7] |
HAN S W, BANG J, KO S H, et al. Variation of nitrogen species in zeolite-templated carbon by low-temperature carbonization of pyrrole and the effect on oxygen reduction activity. Journal of Materials Chemistry A, 2019,7:8353-8360.
DOI URL |
| [8] |
LI S Y, GU Q Q, CAO N, et al. Defect enriched N-doped carbon nanoflakes as robust carbocatalysts for H2S selective oxidation. Journal of Materials Chemistry A, 2020,8:8892-8902.
DOI URL |
| [9] |
CHOI C H, PARK S H, WOO S I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano, 2012,6(8):7084-7091.
DOI URL |
| [10] |
HE H, HUANG D, TANG Y G, et al. Tuning nitrogen species in three-dimensional porous carbon via phosphorus doping for ultra-fast potassium storage. Nano Energy, 2019,57:728-736.
DOI URL |
| [11] |
TO J W F, HE J J, MEI J G, et al. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. Journal of The American Chemical Society, 2016,138(3):1001-1009.
DOI URL |
| [12] |
SU F B, TIAN Z Q, POH C K, et al. Pt nanoparticles supported on nitrogen-doped porous carbon nanospheres as an electrocatalyst for fuel cells. Chemistry of Materials, 2010,22:832-839.
DOI URL |
| [13] |
LIU R L, SHI Y F, WAN Y, et al. Triconstituent co-assembly to ordered mesostructured polymer-silica and carbon-silica nanocomposites and large-pore mesoporous carbons with high surface areas. Journal of The American Chemical Society, 2006,128:11652-11662.
DOI URL |
| [14] |
FERRARI A C, ROBERTSON J. Interpretation of Raman spectra of disordered and amorphous carbon. Physical Review B, 2000,61(20):14095-14107.
DOI URL |
| [15] |
XU F, SUN P, QIAN M, et al. Variable texture few-layer ordered macroporous carbon for high-performance electrochemical capacitors. Journal of Materials Chemistry A, 2017,5:25171-25176.
DOI URL |
| [16] |
WANG H B, MAIYALAGAN T, WANG X. Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and its potential applications. ACS Catalysis, 2012,2(5):781-794.
DOI URL |
| [17] |
SUN J, WANG L, SONG R, et al. Enhancing pyridinic nitrogen level in graphene to promote electrocatalytic activity for oxygen reduction reaction. Nanotechnology, 2016,27:055404.
DOI URL |
| [18] |
SUN H, QUAN H Y, PAN M H, et al. Nitrogen-doped hierarchically structured porous carbon as a bifunctional electrode material for oxygen reduction and supercapacitor. Journal of Alloys and Compounds, 2020,826:154208.
DOI URL |
| [19] |
DEKA N, BARMAN J, KASTHURI S, et al. Transforming waste polystyrene foam into N-doped porous carbon for capacitive energy storage and deionization applications. Applied Surface Science, 2020,511:145576.
DOI URL |
| [20] |
DU J, CHEN A B, LIU L, et al. N-doped hollow mesoporous carbon spheres prepared by polybenzoxazines precursor for energy storage. Carbon, 2020,160:265-272.
DOI URL |
| [21] |
WANG Y Z, LIU Y X, WANG D H, et al. Free-standing honeycomb-like N doped carbon foam derived from coal tar pitch for high-performance supercapacitor. Applied Surface Science, 2020,506:145014.
DOI URL |
| [22] |
HUO S L, ZHANG X L, LIANG B L, et al. Synthesis of interconnected hierarchically porous carbon networks with excellent diffusion ability based on NaNO3 crystal-assisted strategy for high performance supercapacitors. Journal of Power Sources, 2020,450:227612.
DOI URL |
| [23] |
YUKSEL R, BUYUKCAKIR O, PANDA P K, et al. Necklace-like nitrogen doped tubular carbon 3D frameworks for electrochemical energy storage. Advanced Functional Materials, 2020,30(10):1909725.
DOI URL |
| [1] | 杨恩东, 李宝乐, 张珂, 谭鲁, 娄永兵. ZnCo2O4-ZnO@C@CoS核壳复合材料的制备及其在超级电容器中的应用[J]. 无机材料学报, 2024, 39(5): 485-493. |
| [2] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [3] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [4] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| [5] | 李泽晖,谭美娟,郑元昊,骆雨阳,经求是,蒋靖坤,李明杰. 导电金属有机骨架材料在超级电容器中的应用[J]. 无机材料学报, 2020, 35(7): 769-780. |
| [6] | 王佳琦, 庞宏伟, 唐昊, 于淑君, 朱洪涛, 王祥学. 碳热还原法制备的碳载零价铁对水中U(VI)的去除研究[J]. 无机材料学报, 2020, 35(3): 373-380. |
| [7] | 胡丽芳,刘柳,何杰,孙志鹏,陈小平. Ni-Ti-LDHs纳米片对阿司匹林的负载与缓释[J]. 无机材料学报, 2020, 35(2): 165-172. |
| [8] | 陈钧,马培华,张诚,劳伦·鲁尔曼,吕耀康. 新型多功能无机/有机复合薄膜的制备及电化学性能研究[J]. 无机材料学报, 2020, 35(2): 217-223. |
| [9] | 费明婕, 张任平, 朱归胜, 俞兆喆, 颜东亮. 磷酸根掺杂MnFe2O4及其赝电容特性[J]. 无机材料学报, 2020, 35(10): 1137-1141. |
| [10] | 丁卓峰, 杨永强, 李在均. 组氨酸功能化碳点/石墨烯气凝胶的制备及超级电容器性能[J]. 无机材料学报, 2020, 35(10): 1130-1136. |
| [11] | 马亚楠, 刘宇飞, 余晨旭, 张传坤, 罗时军, 高义华. 不同横向尺寸单层Ti3C2Tx纳米片的制备及其电化学性能研究[J]. 无机材料学报, 2020, 35(1): 93-98. |
| [12] | 李腾飞, 黄璐君, 闫旭东, 刘庆雷, 顾佳俊. 碳化钛/椴木多孔碳复合材料用于超级电容器性能的研究[J]. 无机材料学报, 2020, 35(1): 126-130. |
| [13] | 张天宇, 崔聪, 程仁飞, 胡敏敏, 王晓辉. 同步氨化/碳化法制备MXene/C平面多孔复合电极[J]. 无机材料学报, 2020, 35(1): 112-118. |
| [14] | 许伟佳, 邱大平, 刘诗强, 李敏, 杨儒. 用于高性能超级电容器电极的栓皮栎基多孔活性炭的制备[J]. 无机材料学报, 2019, 34(6): 625-632. |
| [15] | 刘伟, 郑凯, 王东红, 雷忆三, 范怀林. Co3O4纳米线阵列@活性炭纤维复合材料的水热合成及电化学应用[J]. 无机材料学报, 2019, 34(5): 487-492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||