无机材料学报 ›› 2019, Vol. 34 ›› Issue (5): 502-508.DOI: 10.15541/jim20180338 CSTR: 32189.14.10.15541/jim20180338
所属专题: 离子电池材料
罗世强,郑春满( ),孙巍巍,谢威,柯剑煌,刘双科,洪晓斌,李宇杰,许静
),孙巍巍,谢威,柯剑煌,刘双科,洪晓斌,李宇杰,许静
收稿日期:2018-07-23
修回日期:2018-11-26
出版日期:2019-05-20
网络出版日期:2019-05-14
作者简介:罗世强(1992-), 男, 硕士研究生. E-mail:ltlsqiang@163.com
基金资助:
Shi-Qiang LUO,Chun-Man ZHENG( ),Wei-Wei SUN,Wei XIE,Jian-Huang KE,Shuang-Ke LIU,Xiao-Bin HONG,Yu-Jie LI,Jing XU
),Wei-Wei SUN,Wei XIE,Jian-Huang KE,Shuang-Ke LIU,Xiao-Bin HONG,Yu-Jie LI,Jing XU
Received:2018-07-23
Revised:2018-11-26
Published:2019-05-20
Online:2019-05-14
Supported by:摘要:
以ZIF-67为模板制备了一系列具有不同金属Co负载量的S/Co-NC复合材料, 并将其应用于锂-硫电池正极中进行电化学性能研究。采用扫描电镜(SEM)和透射电镜(TEM)对Co-NC材料的多面体形貌及多孔结构进行表征; 采用X射线衍射(XRD)分析了Co-NC中金属Co的结晶状态; 采用氮气吸脱附方法分析了Co-NC材料的比表面积及孔结构。研究表明, 当刻蚀时间为48 h, 即Co含量为15.93wt%时, 复合硫正极呈现出最佳的循环性能以及倍率性能, 在0.2C电流密度下从第50圈到200圈循环的容量保持率为94.84%, 5.0C高倍率下的放电比容量为718.8 mAh?g -1。
中图分类号:
罗世强, 郑春满, 孙巍巍, 谢威, 柯剑煌, 刘双科, 洪晓斌, 李宇杰, 许静. ZIF-67衍生Co-NC多孔碳材料的可控制备及其在锂-硫二次电池中的应用研究[J]. 无机材料学报, 2019, 34(5): 502-508.
Shi-Qiang LUO, Chun-Man ZHENG, Wei-Wei SUN, Wei XIE, Jian-Huang KE, Shuang-Ke LIU, Xiao-Bin HONG, Yu-Jie LI, Jing XU. Controllable Preparation of Co-NC Nanoporous Carbon Derived from ZIF-67 for Advanced Lithium-sulfur Batteries[J]. Journal of Inorganic Materials, 2019, 34(5): 502-508.
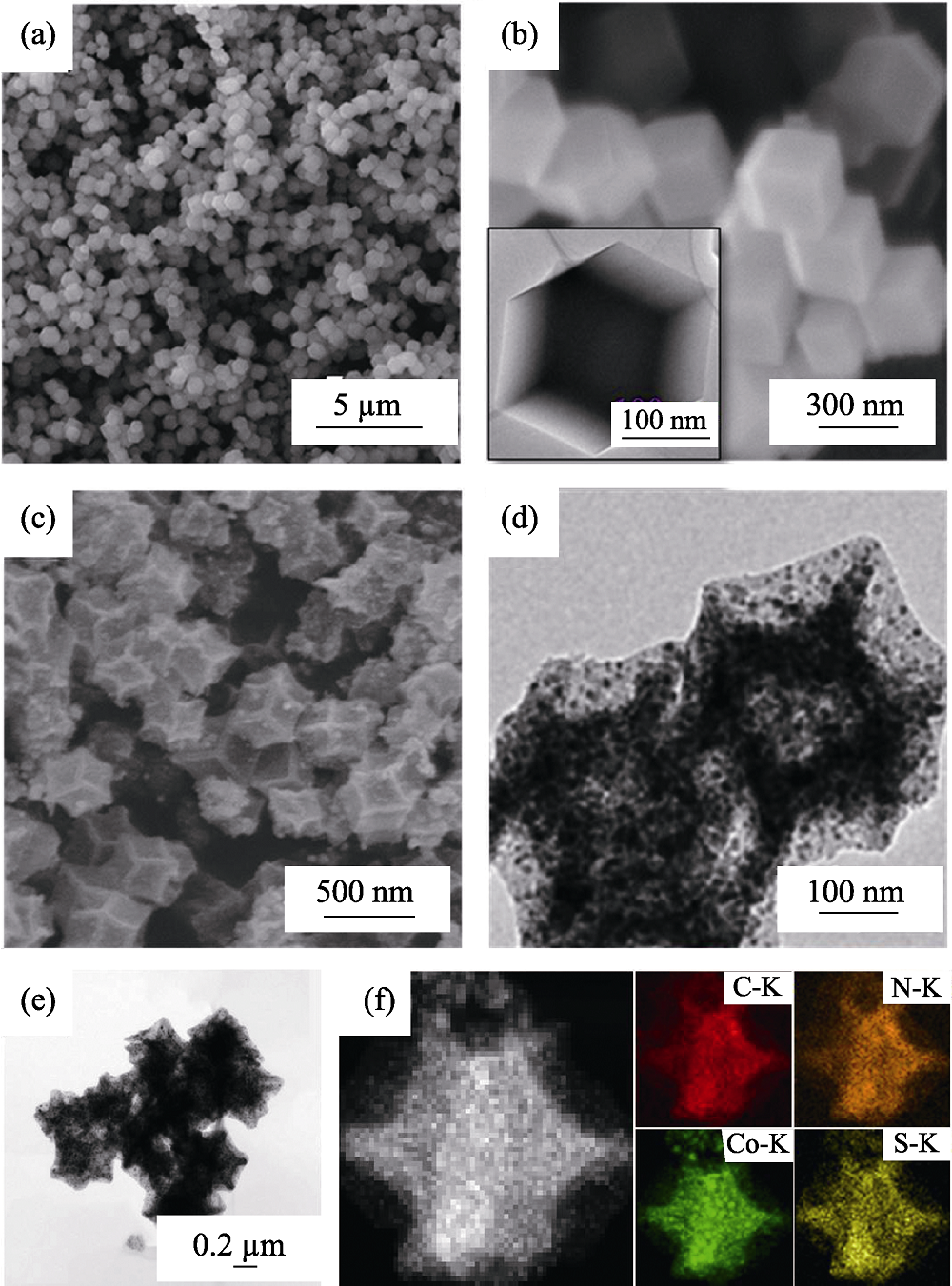
图2 (a~b) ZIF-67前驱体和(c~d) Co-NC(48 h)的(a,c) SEM、(b,d) TEM照片; S/Co-NC复合材料的(e) TEM照片和(f)EDS元素面扫描图
Fig. 2 (a,c) SEM and (b,d) TEM images of (a-b) ZIF-67 precursors and (c-d) Co-NC (48 h); (e) TEM image and (f) EDS elemental mappings of S/Co-NC (48 h) composite

图3 (a) ZIF-67前驱体、(b) Co-NC(0~48 h)以及(c) S/Co-NC (48 h)复合材料的XRD图谱
Fig. 3 XRD patterns of (a) ZIF-67 precursor, (b) Co-NC(0~ 48 h) and (c) S/Co-NC(48 h) composites
| Sample | BET surface area/(m2?g-1) | Pore diameter /nm | Pore volume /(cm3?g-1) |
|---|---|---|---|
| ZIF-67 | 1481.22 | 1.9919 | 0.7252 |
| Co-NC(0 h) | 272.15 | 8.2433 | 0.5608 |
| Co-NC(6 h) | 301.73 | 8.7121 | 0.6571 |
| Co-NC(24 h) | 344.41 | 9.6929 | 0.7016 |
| Co-NC(48 h) | 360.50 | 9.7984 | 0.8124 |
表1 ZIF-67和Co-NC复合材料的BET结果
Table 1 BET results of ZIF-67 and Co-NC composites
| Sample | BET surface area/(m2?g-1) | Pore diameter /nm | Pore volume /(cm3?g-1) |
|---|---|---|---|
| ZIF-67 | 1481.22 | 1.9919 | 0.7252 |
| Co-NC(0 h) | 272.15 | 8.2433 | 0.5608 |
| Co-NC(6 h) | 301.73 | 8.7121 | 0.6571 |
| Co-NC(24 h) | 344.41 | 9.6929 | 0.7016 |
| Co-NC(48 h) | 360.50 | 9.7984 | 0.8124 |

图4 ZIF-67前驱体和Co-NC(0~48 h)样品的(a)氮气吸脱附等温线、(b)孔径分布曲线以及(c) Co-NC(0~48 h)复合材料随刻蚀时间延长BET比表面积及孔体积的变化曲线
Fig. 4 (a) N2 adsorption-desorption isotherms and (b) pore size distributions of ZIF-67 and Co-NC(0~48 h) samples, (c) curves of variation trend of BET surface area and pore volume of Co-NC composites with increased etching time
| Sample | Co-NC(0 h) | Co-NC(6 h) | Co-NC(24 h) | Co-NC(48 h) |
|---|---|---|---|---|
| Co/wt% | 37.55 | 23.29 | 16.24 | 15.93 |
| N/wt% | 6.68 | 8.36 | 8.79 | 8.78 |
| N/C | 0.1070 | 0.1089 | 0.1056 | 0.1045 |
表2 Co-NC复合材料中各元素质量含量及N/C比
Table 2 Elemental content of Co and N in Co-NC composites and N/C ratio data
| Sample | Co-NC(0 h) | Co-NC(6 h) | Co-NC(24 h) | Co-NC(48 h) |
|---|---|---|---|---|
| Co/wt% | 37.55 | 23.29 | 16.24 | 15.93 |
| N/wt% | 6.68 | 8.36 | 8.79 | 8.78 |
| N/C | 0.1070 | 0.1089 | 0.1056 | 0.1045 |

图6 不同Co含量的S/Co-NC(0~48 h)电极的(a) CV曲线和(b)恒电流充放电曲线
Fig. 6 (a) CV curves and (b) discharge-charge profiles of S/Co-NC(0~48 h) electrodes with different Co contents
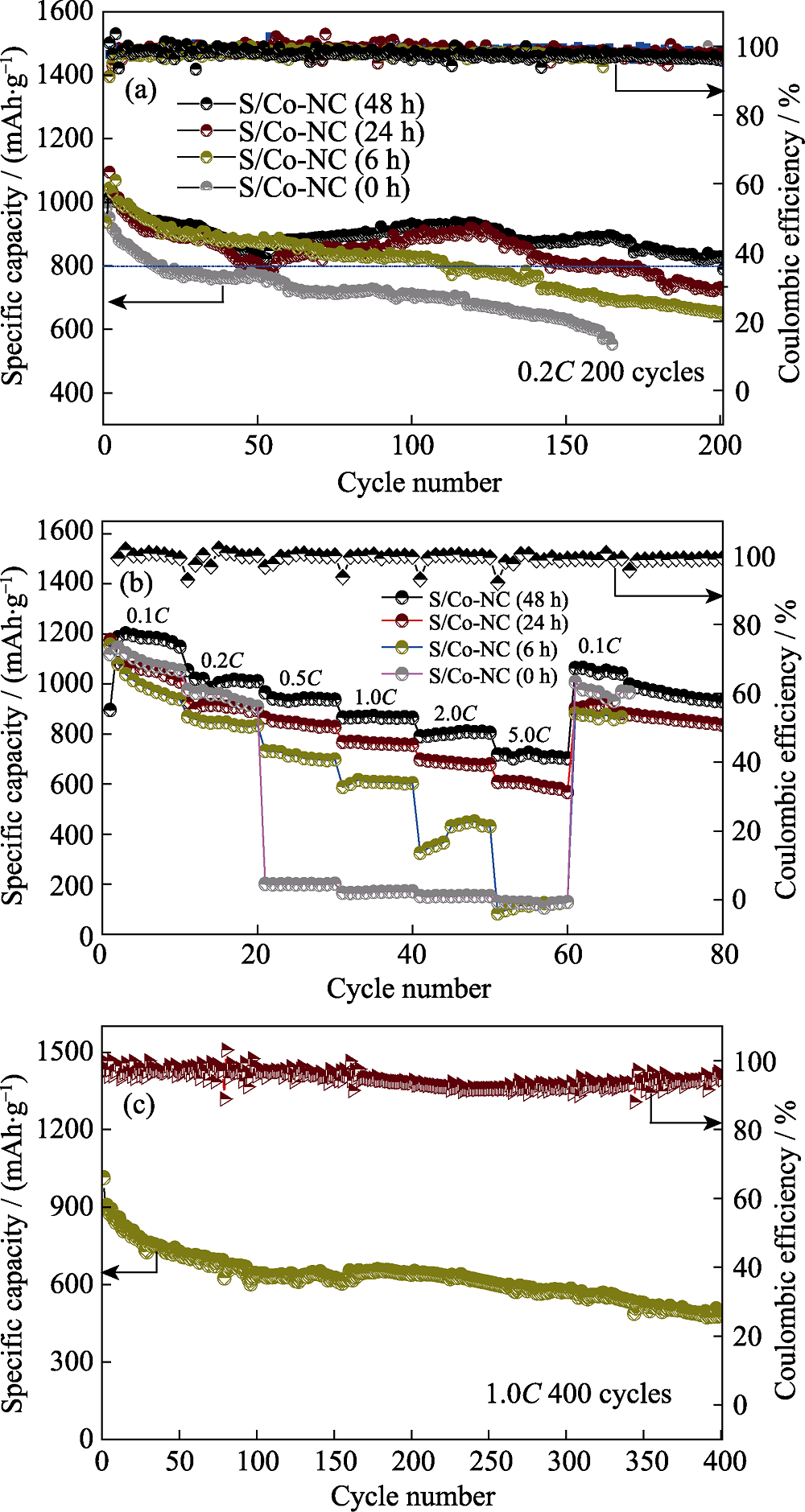
图7 不同Co含量的S/Co-NC(0~48 h)电极的(a)循环性能曲线、(b)倍率性能曲线以及(c)S/Co-NC(48 h)电极在1.0C电流密度下的长周期循环性能曲线
Fig. 7 (a) Cycle performances and (b) rate capabilities of S/Co-NC(0~48 h) electrodes with different Co contents; (c) Long-cycle performance of S/Co-NC(48 h) at 1.0C current density
| [1] | DWNG NAN-PING, MA XIAO-MIN, RUAN YAN-LI , et al. Research and prospect of lithium-sulfur battery aystem. Progress in Chemistry, 2016,28(9):1435-1454. |
| [2] | ZHAO X, CHERUVALLY G, KIM C , et al. Lithium/sulfur secondary batteries: a review. Journal of Electrochemical Science and Technology, 2016,7(2):97-114. |
| [3] | LI FU-QIAO, ZHU ZE-HUA . Development of lithium/sulfur battery and its facing challenge. Chinese Journal of Power Sources, 2016,40(5):1142-1144. |
| [4] |
FOTOUHI A, AUGER DJ, PROPP K , et al. A review on electric vehicle battery modelling: from lithium-ion toward lithium-sulphur. Renewable & Sustainable Energy Reviews, 2016,56:1008-1021.
DOI URL |
| [5] |
KANG W, DENG N, JU J , et al. A review of recent developments in rechargeable lithium-sulfur batteries. Nanoscale, 2016,8(37):16541-16588.
DOI URL PMID |
| [6] |
DIAO Y, XIE K, XIONG S Z , et al. Shuttle phenomenon-the irreversible oxidation mechanism of sulfur active material in Li-S battery. Journal of Power Sources, 2013,235:181-186.
DOI URL |
| [7] | WANG J L, HE Y S, YANG J . Sulfur-based composite cathode materials for high-energy rechargeable lithium batteries. Advanced Materials, 2015,27(3):569-575. |
| [8] |
SEH Z W, SUN Y, ZHANG Q , et al. Designing high-energy lithium-sulfur batteries. Chemical Society Reviews, 2016,45(20):5605-5634.
DOI URL PMID |
| [9] |
PANG Q, LIANG X, KWOK C Y , et al. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nature Energy, 2016,1:71-83.
DOI URL |
| [10] | PENG H J, HOU T Z, ZHANG Q , et al. Strongly coupled interfaces between a heterogeneous carbon host and a sulfur-containing guest for highly stable lithium-sulfur batteries: mechanistic insight into capacity degradation. Advanced Materials Interfaces, 2014,1(7):1-10. |
| [11] | LI G, SUN J, HOU W , et al. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high- performance lithium-sulfur batteries. Nature Communications, 2016, 7: 10601-1-10. |
| [12] |
XU Y, WEN Y, ZHU Y , et al. Confined sulfur in microporous carbon renders superior cycling stability in Li/S batteries. Advanced Functional Materials, 2015,25(27):4312-4320.
DOI URL |
| [13] |
CHONG WG, HUANG J Q, XU Z L , et al. Lithium-sulfur battery cable made from ultralight,flexible graphene/carbon nanotube/ sulfur composite fibers. Advanced Functional Materials, 2017, 27(4): 1604815-1-10.
DOI URL |
| [14] | XIA W, MAHMOOD A, ZOU R , et al. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy & Environmental Science, 2015,8(7):1837-1866. |
| [15] |
YANG S J, KIM T, IM J H , et al. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chemistry of Materials, 2012,24(3):464-470.
DOI URL |
| [16] | XIA B Y, YAN Y, LI N , et al. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nature Energy, 2016,1(1):15006-15012. |
| [17] |
XIA Y, FANG R, XIAO Z , et al. Confining sulfur in N-doped porous carbon microspheres derived from microalgaes for advanced lithium-sulfur batteries. ACS Applied Materials & Interfaces, 2017,9:41339-41446.
DOI URL PMID |
| [18] |
LI Y, FAN J, ZHANG J , et al. A honeycomb-like Co@N-C composite for ultrahigh sulfur loading Li-S batteries. ACS Nano, 2017,11(11):11417-11424.
DOI URL PMID |
| [19] | KHAN IA, NASIM F, CHOUCAIR M , et al. Cobalt oxide nanoparticle embedded N-CNTs: lithium ion battery applications. RSC Advances, 2016,6(2):1129-1135. |
| [20] | ZHANG H, ZHAO W, ZOU M , et al. 3D,mutually embedded MOF@carbon nanotube hybrid networks for high-performance lithium-sulfur batteries. Advanced Energy Materials, 2018, 19: 1800013-1-11. |
| [21] |
LI Y J, FAN M F, ZHENG M S , et al. A novel synergistic composite with multi-functional effects for high-performance Li-S batteries. Energy & Environmental Science, 2016,9:1998-2004.
DOI URL |
| [22] |
VIZINTIN A, CHABNANE L, TCHERNYCHOVA E , et al. The mechanism of Li2S activation in lithium-sulfur batteries: can we avoid the polysulfide formation? Journal of Power Sources, 2017,344:208-217.
DOI URL |
| [1] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [2] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [3] | 梁凤青, 温兆银. 固态锂电池用MOF/聚氧化乙烯复合聚合物电解质[J]. 无机材料学报, 2021, 36(3): 332-336. |
| [4] | 曾凡鑫, 刘创, 曹余良. 去合金化制备具有高循环稳定性的纳米多孔Sb/MCNT储钠负极材料[J]. 无机材料学报, 2021, 36(11): 1137-1144. |
| [5] | 肖民,邢如月,姚寿广,程杰,申亚举,杨裕生. Mn掺杂Ni(OH)2的合成及电化学性能研究[J]. 无机材料学报, 2019, 34(7): 703-708. |
| [6] | 李勇, 何玮鑫, 郑芯月, 于胜兰, 李海同, 黎弘毅, 张蓉, 王雨. 水系钠离子电池普鲁士蓝正极材料的制备与电化学性能研究[J]. 无机材料学报, 2019, 34(4): 365-372. |
| [7] | 赛喜雅勒图, 王雪莹, 顾庆文, 夏永高, 刘兆平, 何杰. 非化学计量比调控高电压尖晶石正极材料电化学性能研究[J]. 无机材料学报, 2018, 33(9): 993-1000. |
| [8] | 孟祥鲁, 霍翰宇, 郭向欣, 董绍明. 薄膜厚度对α-SiOx薄膜负极电化学性能的影响[J]. 无机材料学报, 2018, 33(10): 1141-1146. |
| [9] | 刘双宇, 徐 丽, 陈 新, 韩 钰, 刘海镇, 盛 鹏, 王 博, 赵广耀. 石墨烯负载团簇结构CoFe2O4及其电化学储锂性[J]. 无机材料学报, 2017, 32(9): 904-908. |
| [10] | 杨 桃, 李 肖, 田晓冬, 宋 燕, 刘占军, 郭全贵. 锂离子电池负极材料Si@C/SiOx的制备及其电化学性能[J]. 无机材料学报, 2017, 32(7): 699-704. |
| [11] | 李 想, 葛武杰, 王 昊, 瞿美臻. 高镍系三元层状氧化物正极材料容量衰减机理的研究进展[J]. 无机材料学报, 2017, 32(2): 113-121. |
| [12] | 同艳维, 张雪峰, 方民宪. 镍氢电池负极材料V2Ti0.5Cr0.5Ni1-xMox(x=0.02~0.08)的结构和电化学性能[J]. 无机材料学报, 2016, 31(2): 148-152. |
| [13] | 郭德超, 曾燮榕, 邓 飞, 邹继兆, 盛洪超. 碳纳米管/微膨石墨复合负极材料的制备及电化学性能研究[J]. 无机材料学报, 2012, 27(10): 1035-1041. |
| [14] | 樊军良,潘洪革,高明霞,林燕,刘继强. 无水溶胶-凝胶法制备LiFePO4/C电极材料及其结构和电化学性能[J]. 无机材料学报, 2007, 22(6): 1032-1036. |
| [15] | 吕东生,李伟善,唐仁衡,肖方明. 双辊淬冷法制备稀土镍基贮氢合金及其晶粒尺寸和电化学性能[J]. 无机材料学报, 2005, 20(4): 859-863. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||