天然骨组织是一种高度动态、复杂的血管化组织, 能在整个生命过程中不断重塑[1]。尽管骨组织具有一定程度的自愈能力, 但诸如创伤、感染、肿瘤切除和关节翻修等都可能会造成超出自身愈合能力的骨缺损, 并延长骨愈合和功能恢复所需时间[2]。在这些情况下, 通常需要介入性手术治疗, 即使用骨移植物来固定和促进骨再生。临床常用的骨修复材料包括自体骨、同种异体骨、人工骨材料等[3-4]。“自体骨”是骨修复的金标准材料, 但患者供骨数量有限, 且易导致取骨区各种并发症[5]。同种异体骨虽能解决骨源有限的问题, 但存在疾病传播、免疫反应及骨吸收风险[6]。生物材料(如骨水泥、金属、生物活性陶瓷等)广泛应用于临床骨组织修复。其中, 金属植入物具有较好的稳定性, 主要用作固定装置[7]。聚合物材料如PMMA骨水泥既可作为惰性骨填充材料单独使用, 亦可对金属植入物进行加固[8]。生物活性陶瓷, 如磷酸钙陶瓷和生物活性玻璃等, 主要以块体、涂层和粉体形态用于骨组织修复, 临床常用于非承力部位如上肢骨的修复、骨移植和骨增强的空隙填充[9]。这些生物材料虽然克服了自体骨和同种异体骨的不足, 但其修复效率和修复质量仍显不足。
在骨组织再生过程中, 相关细胞的快速启动、定向分化以及微环境下营养物质输送是决定骨组织再生修复速度的重要因素[10]。因此, 开发具有快速调控细胞/组织生物学响应、高骨再生速率的生物材料是缩短临床治疗时间、降低治疗费用、加速大面积和难愈合骨缺损修复的关键, 也是国内外学者致力研究的方向。天然骨组织具有从微观纳米尺度到宏观尺度的复杂层次结构, 能够产生高机械强度和独特生物学特性[11](图1)。有鉴于此, 研究人员通过模拟天然骨组织微纳米结构特征构建了新型仿生微纳米结构材料, 以促进细胞对特异性蛋白分子的募集、引导细胞取向, 并促进细胞的黏附、增殖及成骨分化, 进而加速骨组织的再生修复效率[12-13]。亦有研究指出通过优化生物材料表面微纳米结构的形状和尺寸, 能够进一步调控微环境下材料吸附蛋白的种类和浓度, 为细胞提供类似天然组织的微环境, 从而提高微纳米结构生物材料的成骨性能[14]。此外, 研究人员还对微纳米形貌特征调控细胞命运的分子机制进行了深入研究, 阐述了微纳米特征结构作用下生物材料调节细胞行为(如黏附、迁移、增殖和分化)的作用途径以及分子机制, 并取得了一定进展[15]。因此, 本文围绕医用骨组织再生修复生物材料, 分别从微纳米结构金属、聚合物和生物活性陶瓷三个方面的研究进展进行综述, 并探讨相关的成骨作用机制, 从而为骨组织再生修复提供新的研究方向, 探寻新的骨缺损再生修复途径。
图1
图1
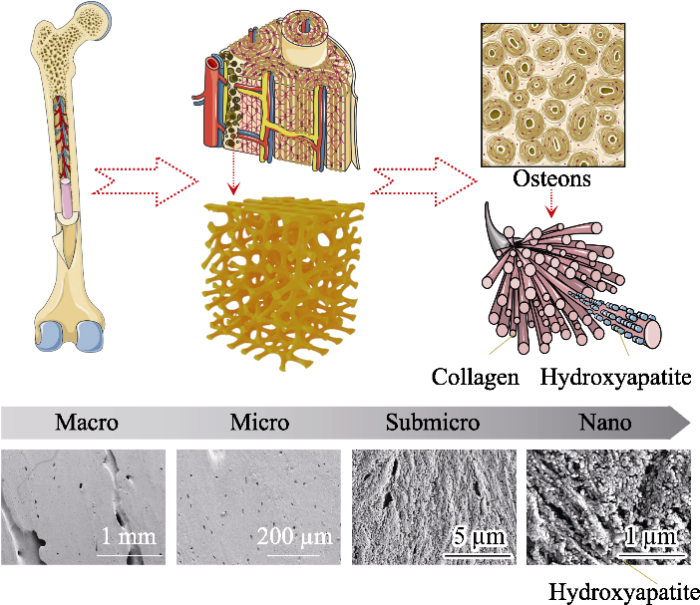
天然骨组织的层次结构模式图和人股骨骨干部皮质骨扫描电镜照片[1,11]
Fig. 1
Schematic diagram of bone hierarchical structural organization (up part) and scanning electron microscope images (bottom part) of the cortical bone specimens located at human femoral diaphysis[1,11]
In bone tissue, macroscale arrangements involve both compact/ cortical bone at the surface and spongy/trabecular bone in the interior. Compact bone is composed of osteons and Haversian canals, which surrounded by blood vessels. Osteons have a lamellar structure, with individual lamella consisting of fibers arranged in geometrical patterns. The fibers comprise several mineralized collagen fibrils, composed of collagen protein molecules formed from three chains of amino acids and nanocrystals of hydroxyapatite, and linked by an organic phase to form fibril arrays
1 微纳米结构骨修复材料的作用机制
研究表明将微纳米层次结构引入植入物表面后, 能更好地模拟天然骨组织的结构, 从而实现快速骨融合。在不添加药物和生长因子的情况下, 这种分层微纳米结构能够刺激细胞浸润增殖、营养/废物运输、骨骼向内生长以及血管形成[16]。微纳米分级结构可以通过调控诸多生物化学信号通路如Wnt/β-连环蛋白(Wnt/β-catenin)、蛋白激酶 B(AKT)、血管动蛋白130/Yes相关蛋白(AMOT130/YAP)等, 以及骨免疫微环境协同刺激细胞向骨细胞系分化, 进而促进新骨形成[17⇓-19]。另有研究发现微纳米结构不仅能够显著抑制破骨细胞的形成和活性[20], 还能够调控巨噬细胞黏附状态, 促进巨噬细胞从表型M1向M2极化, 从而构建促进愈合的免疫环境, 进一步促进成骨和血管生成的相关基因表达[21]。这些研究表明, 特定的微纳米层次结构不仅能够通过调节免疫微环境促进成骨和血管生成, 同时还能抑制破骨细胞形成和功能。以此为基础, 深入探索材料表面微纳米结构调控细胞功能及其体内成骨性能的作用机制, 将可为新型生物功能材料的设计提供更精确的指导。
1.1 微纳米结构生物材料对成骨的作用
越来越多的研究表明, 微纳米结构生物材料在骨组织工程中具有巨大的潜力, 能为骨组织修复提供有利的微环境, 引导细胞取向, 促进细胞增殖、黏附和成骨分化, 进而增强骨再生[12]。并且与单个微纳米形貌相比, 分层微纳米形貌具有综合优势, 即微尺度结构能加强骨与植入物之间的联锁(Interlocking), 而纳米尺度结构能促进蛋白质吸附、细胞黏附和最终的骨整合[22]。例如, 研究发现相较于原始选择性激光熔化钛表面, 由阳极氧化产生微管/纳米管(TNT)和由碱热处理形成微管/纳米网(TNN)钛表面的粗糙度降低, 亲水性增加, 碱性磷酸酶(ALP)活性和成骨相关基因的表达提高[23]。Li等[24]采用溶剂流延和静电纺丝法制备了由聚乳酸-羟基乙酸共聚物(PLGA)和微纳米生物活性玻璃(MNBG)组成的新型双层膜(MNBG/PLGA), 并发现该双层膜能够促进成骨相关基因RUNX2、骨桥蛋白的表达, 进而增强骨再生。Zhang等[17]利用数字光处理(DLP)打印与原位晶体生长技术构建微纳米结构β-磷酸三钙支架, 其能够促进大鼠骨髓间充质干细胞(BMSCs)的黏附和增殖, 并通过靶向丝裂原活化蛋白激酶(MAPK)、信号传导及转录激活子(STAT)和AKT信号通路显著促进BMSCs的成骨分化。体内实验结果进一步表明微纳米拓扑结构支架能够有效调节缺损部位的微环境, 加速修复骨缺损。
其次, 研究发现微纳米形貌(Micro/nano-topography, MNT)能够影响肌动蛋白细胞骨架, 并且在肌动蛋白细胞骨架动力学中起关键作用的RhoGTPases家族成员Rac1还可通过调控MAPK通路活性在MNT的成骨分化调控中发挥重要作用[25]。进一步, Long等[26]研究发现在金属钛上结合分级的宏-微-纳米粗糙度能够使成熟成骨细胞/骨细胞呈现典型的星状形态, 快速随机迁移, 并促进间充质干细胞的成骨分化。另外, 表/界面纳米结构可以通过调节生长因子的构象来提高生物活性, 从而提高促成骨分化能力, 促进骨再生[27]。Li等[28]研究了微纳米羟基磷灰石生物陶瓷对骨形态发生蛋白2(BMP2)结构的影响以及对骨髓基质细胞反应的影响, 结果发现, 与亚微米级结晶颗粒的材料相比, 微纳米结构羟基磷灰石生物陶瓷表现出较高的粗糙度、良好的亲水性和较强的机械性能, 并能够维持BMP2的构象, 促进细胞的黏附和成骨分化。体内实验进一步表明该微纳米结构陶瓷具有更为优异的骨诱导性[29]。同样地,Zhao等[30]通过水热法和模板法制备了微纳米结构羟基磷灰石生物陶瓷, 并研究了表面结构对整合素表达、BMP2信号通路和细胞间通讯的影响。结果发现微纳米结构羟基磷灰石生物陶瓷诱导的成骨分化首先通过激活整合素来调节, 然后进一步激活BMP2信号通路和细胞通讯。而激活的BMP2反过来可以激活整合素和间隙连接Cx43相关的细胞通讯, 由此推断微纳米结构的不同激活机制可导致整合素激活和成骨的协同刺激效应[30]。
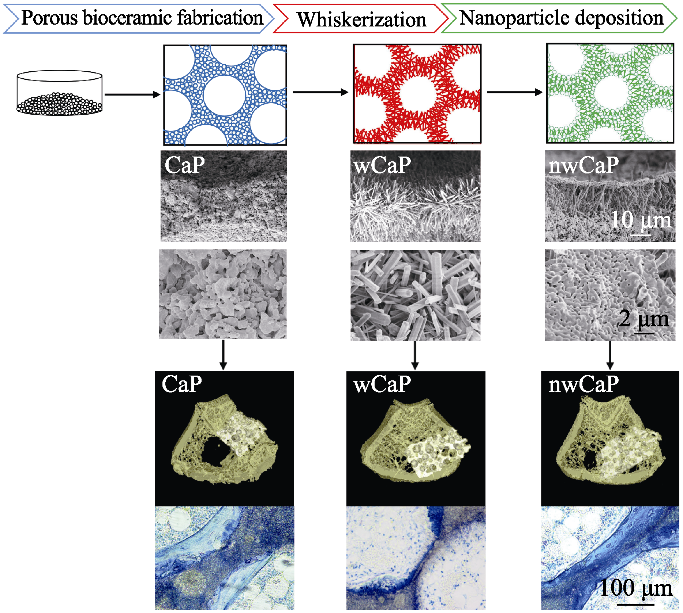
此外, 生物材料的力学性能也是影响成骨的重要因素。理想的骨组织再生生物材料应在力学性能上与天然骨相匹配, 以便在骨再生的初始阶段发挥支持作用, 进而实现骨缺损的完全再生。Xia等[31]通过水热处理构建了微纳米结构磷酸钙生物陶瓷, 发现该微纳米结构磷酸钙陶瓷材料的力学性能得以显著增强, 并能够促进间充质干细胞向骨细胞系分化。本实验室在前期研究中发现, 对多孔磷酸钙陶瓷进行水热处理后, 陶瓷表面能够生长羟基磷灰石晶须, 通过将磷酸钙纳米粒子引入晶须化陶瓷表面, 成功构建了力学增强的微纳米结构磷酸钙(nwCaP)陶瓷[32]。植入比格犬股骨节段性骨缺损后, 其不仅能与宿主骨形成良好的骨整合, 而且在材料内部有大量的新骨生成。进一步将其应用于骨质疏松大鼠骨缺损修复, 发现nwCaP陶瓷材料不仅具备初始力学稳定性, 植入该材料的动物也表现出更低的骨折率、优异的成骨效果和新骨取代率[33](图2)。基因芯片分析结果表明, 该复合陶瓷通过JAK2信号通路选择性上调成纤维细胞生长因子23(FGF23)促进骨形成(图3)。同时研究发现, 在微纳米形貌诱导间充质干细胞分化过程中, 相关物理因素的信号通路被启动, 并参与调控细胞的成骨分化过程。机械应力是物理因素中的重要组成, 启动力学信号通路能调控干细胞的分化、增殖、迁移等生命活动[34]。例如, Liu等[18]以不同孔径的二氧化钛纳米管为基底, 探讨力学信号在微纳米形貌诱导BMSCs成骨分化中的作用, 发现作为力学信号通路的潜在参与者, AMOT130/YAP是介导微纳米形貌诱导BMSCs成骨分化的重要途径。另有研究设计了压电纳米纤维支架, 并基于干细胞与材料之间的动态机械相互作用, 借助细胞迁移力引起压电纤维的机械形变, 产生压电电信号反作用于细胞, 从而调控干细胞命运与组织再生, 证实细胞不仅被动地响应细胞外基质传递的生化和生物物理信号, 也能主动改变周围微环境以满足其需要[35]。
图2
图2
不同表面形貌的传统磷酸钙(CaP)、晶须化磷酸钙(wCaP)和微纳米结构磷酸钙(nwCaP)生物陶瓷制备流程图和成骨效果[33]
Fig. 2
Schematic diagram of preparation process and bone forming ability of traditional calcium phosphate (CaP), whiskered calcium phosphate (wCaP) and micro-/nano-structured calcium phosphate (nwCaP) bioceramics with different surface morphologies[33]
图3
图3
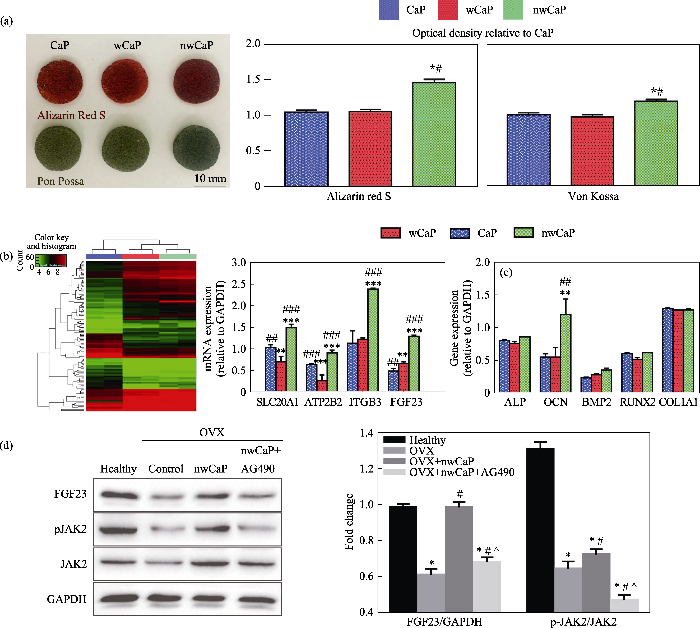
微纳米结构磷酸钙(nwCaP)生物陶瓷诱导成骨所涉及的分子机制研究[33]
Fig. 3
Illustration of the possible molecular mechanism involved in nwCaP bioceramics induced osteogenic effect[33]
(a) Photos of Alizarin Red S and von Kossa stainings; (b) Cluster analysis of genes and quantitative qRT-PCR analysis expressions; (c) Osteogenesis-related gene expression; (d) Representative western blot analysis; OVX: Ovariectomized
1.2 微纳米结构生物材料对破骨的作用
最近的研究不仅集中于微纳米结构生物材料对间充质干细胞的影响, 还致力于阐明这类材料对破骨细胞和破骨前体细胞的作用, 因为破骨细胞不仅能促进生物材料的再吸收, 还能触发成骨细胞的响应[36]。众多研究指出相较于原材料, 微纳米结构生物材料不仅能够显著降低破骨细胞标志物抗酒石酸酸性磷酸酶(TRAP)和破骨细胞生成标志物的基因表达, 还能阻碍破骨细胞的融合和骨吸收活性, 进而促进成骨[36-37]。例如, 与羟基磷灰石材料相比, 微纳米结构羟基磷灰石材料虽不影响破骨前体细胞的黏附, 但能够阻碍破骨前体细胞的融合和再吸收活性, 证明微纳米表面形貌对破骨细胞形成和活性有显著抑制作用[20]。Bai等[21]在钛金属种植体表面构建的微纳米结构二氧化钛纤维样仿生结构网络(Micro/nano-scale, MNS), 能够下调TRAP和组织蛋白酶K(CTSK)的表达。Yu等[23]对比研究了钛表面微管/纳米管(TNT)和微管/纳米网(TNN)的成骨性能, 二者与钛表面(SLM)组相比, TNT组成骨相关基因表达最高, TNN组次之, 而在破骨细胞生成方面, TNN的TRAP活性和破骨细胞生成相关基因表达最低, TNT低于SLM, 但高于TNN。植入体内4 w后, TNT组种植体周围骨百分比(BV/TV)最高, 且其周围的成骨细胞和破骨细胞活性均高于TNN, 表明TNT组骨转换率较高。Chen等[38]通过肌内植入模型研究羟基磷灰石材料诱导的骨形成与非骨部位破骨细胞出现之间的潜在关系, 发现与亚微米尺度羟基磷灰石材料相比, 微纳米结构羟基磷灰石材料能够显著抑制破骨细胞的形成和功能, 表现为细胞融合受到抑制、凋亡增加、特异性基因和蛋白质表达受到抑制、TRAP阳性细胞减少等, 该研究表明生物活性陶瓷的表面结构对破骨细胞的形成具有很大影响, 并且破骨细胞形成可能与其骨诱导能力有关。以上研究证实微纳米结构生物材料在抑制破骨方面的功效, 然而目前对其内含分子机理仍需要进一步探索。
1.3 微纳米结构生物材料对血管再生的作用
在组织修复过程中, 骨生成与血管生成紧密相伴[39]。其中, 促进血管再生的策略包括靶向血管前体细胞、增强内皮化和生物活性材料诱导等[40]。据报道, 微纳米结构生物材料一方面能够促进内皮细胞黏附、增殖, 另一方面还能促进内皮细胞血管生成基因的表达, 进而促进血管生成[41-42]。例如, 研究发现钛种植体表面微纳米尺度的二氧化钛纤维样仿生结构网络能够促进骨髓基质细胞的成骨分化和内皮细胞的血管分化[21]。Yang等[43]发现微纳米网状结构钛金属具有较高的亲水性和中等的粗糙度, 能够通过Src-ROCK信号引导细长巨噬细胞黏附状态, 将巨噬细胞转换为M2表型, 并促进内皮细胞bEnd.3血管生成相关因子(如PDGF、IGF、FGF等)的表达, 体内研究也显示其血管生成和骨形成加快。Tian等[44]研究发现PLGA和MNBG复合多孔支架能促进人脐静脉血管内皮细胞(HUVECs)的黏附和增殖, 并显著增强HUVECs血管生成标记物CD31的表达。Liu等[45]在复合陶瓷β-TCP/CaSiO3上原位构建了微纳米棒杂化羟基磷灰石表面层, 其能够促进小鼠BMSCs的成骨分化和HUVECs血管生成相关基因CD31和血管内皮生长因子(VEGF)的表达, 异位皮下移植的研究结果进一步表明其在植入4 w后促进毛细血管形成和骨再生, 8 w后在远离骨-植入物界面的部位诱导新骨基质形成。还有研究通过光刻结合水热法制备由不同纳米形貌(纳米针、纳米片和纳米棒)和尺寸(4、12和36 µm)微图案组成的不同微纳米结构羟基磷灰石生物陶瓷, 其中具有适当图案尺寸的微纳米分级结构可以促进巨噬细胞向M1或M2极化, 进而改善骨再生[41]。本研究团队曾发现负载适量纳米羟基磷灰石的微纳米结构羟基磷灰石生物陶瓷不仅能够促进骨质疏松区域成骨细胞的增殖和黏附, 还可上调成骨相关因子(ATP2A2和FGF23)的表达, 当植入骨质疏松大鼠临界骨缺损后, 与成骨相关的标志物CD31、EMCN均呈阳性的毛细血管细胞数量也会更多, 骨形成显著增强[16](图4)。
图4
1.4 微纳米结构生物材料对骨免疫调节的作用
骨免疫调节是指对巨噬细胞向促炎症M1和抑炎症M2方向极化免疫反应的操纵, 以产生有害或有益的免疫微环境, 进一步影响骨再生和血管生成[46]。如前所述, 微纳米结构生物材料能够通过调节免疫反应进而驱动血管生成和骨再生。例如, Chen等[47]研究发现纳米结构可为蛋白质吸附以及促进成骨提供较大的表面积, 并且表面纳米孔结构可提供不同的免疫环境, 从而改变巨噬细胞的形状, 诱导骨免疫反应, 进而促进早期骨再生中成骨细胞系的招募和分化。因此, 控制纳米孔的大小或能调节免疫细胞的行为。研究发现与较小孔径(20 nm)相比, 大孔径(200 nm)的巨噬细胞附着较少, 然而其附着的巨噬细胞高度活化, 释放的促炎细胞因子较多, 体内实验结果进一步表明与孔径20 nm的氧化铝相比, 孔径200 nm的氧化铝引起了更强的炎症反应, 原因在于孔径200 nm的氧化铝周围有更多的细胞募集和促炎细胞因子的产生[48-49]。随后的研究表明纳米孔的结构和孔径能够影响免疫细胞的扩散和形状变化, 从而调节自噬通路因子(LC3A/B、Beclin-1、Atg3、ATG7、p62等)的表达和激活, 从而调节免疫细胞的生长和增殖[50]。
研究发现, 钛种植体表面微纳米尺度的二氧化钛纤维样仿生结构网络(Micro/nano-scale, MNS)能够调控巨噬细胞向抑制炎症的M2极化, 抑制促炎症的M1极化, 从而导致炎症相关细胞信号通路下调[21]。另外, 在MNS上也发现了自噬相关基因(ATG5、LC3A/B、P62)上调, 因此该研究证实MNS能够通过调控骨免疫微环境进一步促进骨/血管的生成。Zhang等[46]通过研制光滑钛和微纳米钛金属植入物探讨了巨噬细胞RAW264.7作用下免疫微环境对成骨细胞MC3T3-E1的分化和自噬的影响, 结果发现微纳米钛金属植入物可刺激RAW264.7细胞分化为M2型, 形成抗炎免疫微环境, 以促进成骨细胞的增殖和分化。另外, 抗炎免疫环境能够激活成骨细胞自噬水平, 抑制自噬后成骨标志物表达下调。该研究表明抗炎免疫微环境可以促进成骨细胞增殖和分化, 自噬在这一过程中起重要作用。同样, Yang等[41]通过光刻结合水热法制备微纳米结构羟基磷灰石生物陶瓷, 结果发现适当图案大小的微纳米分级结构能够调节炎症反应, 进而影响人骨髓基质细胞的成骨分化和HUVECs的血管生成能力。
2 微纳米结构生物材料对体内骨组织再生的影响
为满足骨缺损重建的临床需要, 研究人员开发探索了多种人工骨移植替代物, 包括金属植入物、聚合物和生物陶瓷等[51]。其中, 金属植入物如316L不锈钢、钛合金、钴基合金、铌和形状记忆合金等具有较好稳定性、高强度、高断裂韧性、高硬度以及能够通过标准加工程序加工出复杂形状等优点[52]。尽管金属材料具有优异的机械性能, 但其刚度过高、生物活性较差常引发应力屏蔽效应, 并且由于其与骨结合能力弱, 易导致种植体松动[53]。聚合物如聚醚醚酮(PEEK)、聚乳酸(PLA)、聚羟基乙酸(PGA)等被广泛应用于骨组织再生修复, 由于它们具有生物力学适配性好、加工性能优良等特点, 能够较好满足骨组织工程支架材料的需求[54]。然而聚合物生物活性较差, 难以有效实现骨再生, 长期效果欠佳。另外一些合成聚合物的体内降解产物易引发炎性反应, 从而影响其生物相容性和组织修复进程[55]。生物活性陶瓷如磷酸钙生物陶瓷、生物活性玻璃及其相关化合物等具有良好的骨传导性和骨诱导性, 能与周围骨组织形成无纤维组织的直接接触, 引导周围细胞浸润以及组织生长到缺损部位, 与周围组织发生反应, 逐渐形成牢固的化学键合[56]。然而其低断裂韧性、拉伸强度和耐磨性以及脆性严重限制了其在承重部位的应用, 目前主要以块体、涂层和粉体的形式用于临床[57]。因此需要深入了解材料理化性质与其生物学性能之间的构效关系, 并不断优化材料设计以增强植入材料的生物活性及其组织适配性, 改善组织反应, 恢复植入物的早期功能, 并增强长期稳定性。
2.1 微纳米结构金属植入物对骨再生的影响
研究人员发现微纳米结构金属植入物一方面能够增加种植体与周围宿主骨之间的摩擦, 有效避免骨愈合过程中的微移动, 从而提高种植体的初始稳定性, 另一方面能够在一定程度上模拟自然骨的层状结构特征, 调控细胞行为, 改善植入物与宿主骨的整合[58-59]。例如, Zhao等[60]采用酸蚀和阳极氧化方法制备了微纳米结构钛金属, 结果发现其具有更大的比表面积, 在植入体内后能够募集特异性蛋白, 促进蛋白质的吸附。进一步研究发现, 金属钛表面的分级宏-微-纳米粗糙度不仅使成熟成骨细胞/骨细胞呈现出典型的星状形态, 还显著改善了共培养的间充质干细胞的成骨分化[26]。Ueno等[61]通过碱热处理在钛表面构建了簇状、板状和结节状结构的纳米形貌特征, 体内实验结果表明添加纳米形态特征后, 钛种植体固定效果以及种植体的骨接触百分率显著提高。Brånemark等[58]进一步研究发现, 通过拓扑激光改性, 钛种植体表面微纳米形貌和表面氧化物增加, 从而促进了骨-种植体界面的骨形成。此外, Chen等[27]通过选择性激光熔化的方式将纳米孔特征引入到钛表面, 其微细粗糙表面可以作为微一级基底, 进而形成微纳米纹理表面, 从而促进骨再生, 体内研究结果证实与纳米管的无序排列仿生特征相比, 纳米网的骨诱导效果更为优异。也有研究指出经过抛光和酸处理能够分别构建纳米、亚微米和微米表面形貌的生物可吸收锌金属, 其中亚微米和微纹理锌金属表面粗糙度增加有助于迅速减少巨噬细胞的炎症极化和血小板的黏附, 并促进细胞的成骨分化[62]。
对微纳米结构金属植入物的研究, 不只局限于金属材料本身的改性研究, 还包括对其表面涂层材料的结构研究。例如, Wang等[63]在钛合金表面等离子体喷涂碳酸钙材料, 进一步通过水热法将其转化为纳米花簇结构硅酸钙涂层。结果发现该纳米结构不仅降低了硅酸钙的降解速度, 还增强了其表面磷灰石的矿化能力, 从而促进了细胞黏附、增殖和成骨分化。Zhou等[64]通过水热处理制备了锶掺杂微纳米粗糙钛表面(MNT-Sr), 并发现MNT-Sr可通过诱导干细胞迁移和成骨分化, 实现更好的骨整合。另有研究发现在钛植入体表面构建数十纳米的微孔(直径范围为30~50 nm)交错自组装TiO2纳米管不仅能够赋予材料更大的表面能和粗糙度, 更优异的亲水性和更适配的机械性能, 还能够更好地促进成骨细胞附着和生长[65]。Ding等[66]采用阳极氧化结合大气等离子喷涂的方法将直径约为15 nm的钽氧化物沉积在等离子喷涂的微孔钽涂层上, 制备了微纳米结构钽(MNT)涂层, 体外实验结果表明与微孔钽涂层相比, MNT涂层的耐腐蚀性提高了大约一个数量级, 并能够减少释放腐蚀金属离子, 而且MNT涂层能够有效增殖和分化BMSCs。
2.2 微纳米结构聚合物对骨再生的影响
在微纳米尺度下, 松质骨和密质骨均由矿化胶原纤维以纤维束状的形式进行不同排列而组成[67]。聚乳酸(PLA)、聚乙醇酸(PGA)和聚己内酯(PCL)或它们的共聚物(如PLGA、PLLA或PCLL)具有良好的静电纺丝性能、仿细胞外基质的能力以及细胞相容性和生物降解性, 是组织工程中最常用的合成聚合物[68]。因此, 关于微纳米结构聚合物的研究主要聚焦于仿生自然骨组织结构的微纳米纤维支架。例如, Santos等[69]研究了淀粉和聚己内酯复合微纳米纤维支架对人内皮细胞(ECs)的影响, 并发现微纤维网状结构上的纳米网络不仅维持了ECs的结构完整性及细胞间接触, 还能够促进血管生成。另有研究通过双挤出静电纺丝技术将聚乳酸-羟基乙酸共聚物(PLGA)微纤维网状物与胶原(Col)、羟基磷灰石混合制备出微纳米纤维PLGA-Col-HA三维支架[70]。结果发现与未修饰的微纤维PLGA支架和微纳米纤维PLGA/Col支架相比, 微纳米纤维PLGA-Col-HA支架具有较高的生物活性。此外, Gong等[71]将中药淫羊藿苷和抗菌药物盐酸莫西沙星分别引入聚己内酯核和明胶壳中, 通过同轴静电纺丝法制备了兼具成骨和抗菌作用的微纳米骨膜。实验结果表明通过核壳结构和PCL及明胶的降解率不同, 药物释放曲线可以逐步控制, 盐酸莫西沙星的快速释放和淫羊藿苷的持续释放可以有效抑制细菌定植, 同时促进兔桡骨缺损再生。
不仅如此, 众多研究小组还试图通过引入纳米结构(如在聚合物基质中加入纳米颗粒或纳米纤维增强物)来模拟骨的天然纳米复合结构, 从而操纵支架的机械性能(如刚度、强度和韧性等)。例如, Cui等[72]采用熔融纺丝法制备了羟基磷灰石/聚丙交酯-乙交酯复合支架, 结果发现该支架的抗压强度可达6.27 MPa, 接近于人类小梁骨, 而且该微纳米纤维支架还可上调成骨相关基因BMP2和I型胶原的表达, 进而促进兔桡骨缺损修复。Xu等[73]研制了碳纤维增强的聚醚醚酮-纳米羟基磷灰石(PEEK/n-HA/CF)微纳米复合材料, 结果发现该微纳米结构复合材料在体外能显著促进成骨细胞MG-63的增殖和分化, 在体内能促进种植体与宿主骨之间的结合。Li等[74]利用聚多巴胺(PDA)修饰三维打印技术制备的聚己内酯支架表面, 从而实现纳米银(nAg)的富集。研究结果表明与纯PCL支架相比, 制备的微纳米自组装PDA和nAg颗粒的nAg/PDA/PCL复合支架不仅具有减少细菌黏附和抑制细菌增殖的作用, 还表现出更好的矿化骨组织聚集性能。Wu等[75]将氮化硅(SN)微粒与聚乙二醇化PEEK共混后, 利用飞秒激光处理SN/PEKK复合材料(SPC), 构建了表面微纳米结构的SN/PEKK植入物(FSPC)。结果发现与SPC和PEKK相比, FSPC诱导的微纳米结构表面促进了BMSCs黏附、存活和成骨分化, 表现出更强的抑菌活性。进一步植入兔股骨缺损后发现, 与SPC和PEKK相比, FSPC表现出最大的骨-种植体接触和最大的推出力, 证实微纳米结构表面能够显著促进骨整合。Li等[24]采用溶剂流延和静电纺丝法制备了由聚乳酸-羟基乙酸共聚物(PLGA)和微纳米生物活性玻璃(MNBG)组成的粗糙多孔双层膜。结果发现该复合膜具有稳定的力学性能、良好的生物相容性, 并且MNBG还能够促进BMSCs的成骨分化。
2.3 微纳米结构生物活性陶瓷对骨再生的影响
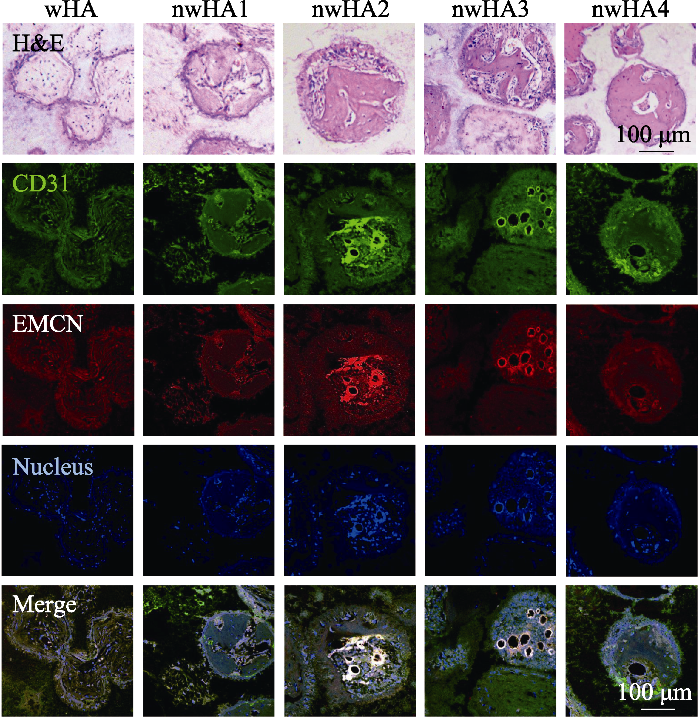
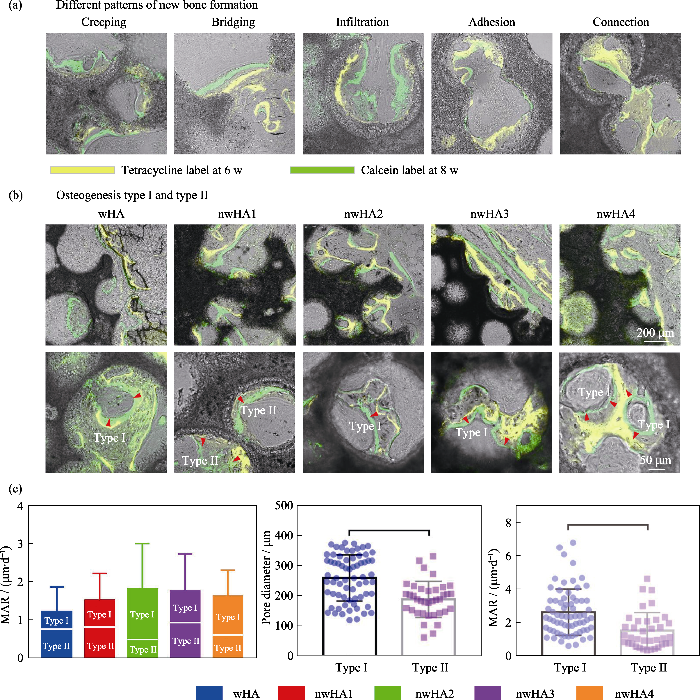
骨组织具有矿化的大孔结构, 其纳米级成分决定其强度, 无机羟基磷灰石(HA)是矿化组分的主要组成部分。由于以HA为主的生物陶瓷的固有脆性和低断裂韧性, 构建多级分层微纳米结构并在纳米尺度上控制其表面形貌仍是一个巨大的挑战。Lin等[76]以(α-磷酸三钙)α-TCP陶瓷作为前驱体, 通过水热反应制备微纳米结构羟基磷灰石陶瓷。结果发现纳米片、纳米棒和微纳米棒状组合三种结构都能够提高材料表面对蛋白质的选择性吸附, 促进细胞黏附、增殖和分化, 而微纳米棒状组合结构的性能优于两种单级纳米结构。Elrayah等[77]通过在水热条件下调节铜离子(Cu2+)的浓度制备了不同微纳米表面的羟基磷灰石支架, 且随着Cu2+浓度增大, 支架的表面由球状转变为花状, 最终形成纳米结构。体外实验结果进一步表明羟基磷灰石支架表面的微纳米结构能够影响内皮细胞的增殖, 其中与花状形态支架共培养的细胞增殖显著增强。体内实验进一步表明羟基磷灰石支架表面的微纳米结构显著影响血管的形成, 而花状支架对血管形成的促进作用最明显。Zhang等[17]将数字光处理(DLP)打印技术与原位晶体生长技术相结合构建了微纳米结构多孔β-磷酸三钙(β-TCP)支架, 结果发现微纳米结构β-TCP支架不仅能够促进BMSCs的黏附和增殖, 还能够促进BMSCs的成骨分化, 其中氢氧化钠处理组的促进作用最强。体内实验结果表明该微纳米结构支架能够通过调节骨微环境促进大鼠颅骨缺损再生修复。本研究团队前期通过调节真空灌注工艺参数控制晶须化羟基磷灰石陶瓷表面羟基磷灰石纳米粒子的负载量, 从而显著提升微纳米结构羟基磷灰石陶瓷的成骨性能[16]。另外, 通过序列荧光标记技术发现在生物陶瓷孔内部存在两种类型(Ⅰ型和Ⅱ型)的新骨形成方式, 在Ⅰ型成骨中新骨形成方向朝向邻近的孔壁, 而在Ⅱ型成骨中新骨形成方向远离邻近的孔壁, 并且在具有较强成骨能力的微纳米结构羟基磷灰石生物陶瓷中主要发生Ⅰ型成骨, 表明其对新骨形成的促进作用较大(图5)。
图5
图5
连续荧光标记评估动态骨形成[16]
Fig. 5
In vivo sequential fluorescence labeling of new bone formation inside porous nwHA bioceramics[16]
(a) Observed patterns of new bone formation (yellow: tetracycline label; green: calcein label); (b) Two types of osteogenesis discovered inside the pore structure of nwHA bioceramics (green indicating CD 31, Red indicating EMCN, and blue indicating nucleus); (c) Comparison of mineral apposition rate (MAR) between different nwHA groups, with statistical analysis of the relationship between osteogenesis type and pore diameter of the bioceramics, and the relationship between osteogenesis type and MAR
其次, Moorthi等[78]报道了纳米生物玻璃陶瓷(nBGC)颗粒的生物活性及促成骨作用, 证实了nBGC颗粒可促进大鼠骨原细胞增殖, 刺激细胞内ERK信号通路及细胞周期蛋白表达, 并能通过成骨相关转录因子RUNX2介导骨原细胞的成骨分化。Lin等[79]研究发现硅酸钙纳米纤维在体外可以刺激BMSCs的成骨分化, 在体内可促进骨再生。Feng等[80]采用3D打印结合水热处理制备了具有中空通道和微纳米表面的硅酸盐基生物陶瓷(AKT-H-N)。除了提高支架的机械强度, 微纳米结构还有利于BMSCs黏附和增殖, 体内实验结果表明空心通道和微纳米结构对骨再生具有协同效应, 植入12 w后能够促进兔股骨缺损处形成新骨。Tang等[81]以介孔生物活性玻璃(MBG)为基体, 采用“黏度控制”和“均匀颗粒增强”多模板工艺制备了负载重组人骨形成蛋白-2(rhBMP-2)的宏微纳米三模态多孔支架(Trimodal MBG scaffold, TMS)。结果发现该支架不仅具有良好的结构稳定性和机械强度, 还具有良好的骨传导性、骨诱导性、rhBMP-2缓释性以及生物降解性。Hu等[82]采用溶胶-凝胶法结合模板法制备的微纳米结构生物活性玻璃(MNBG), 不仅表现出较高的磷灰石形成能力和良好的生物相容性, 还能促进人牙髓细胞(HDPC)的增殖与分化。
3 结论与展望
在组织工程中, 生物材料能够为细胞的黏附、增殖和分化以及组织的形成和生长提供机械支撑和内部空间[83]。当生物材料植入体内后, 首先发生的是细胞/组织表面受体与材料表面的相互识别和作用, 进而引发特异性生物学反应、激活相关基因表达, 最终影响组织再生[84]。这一过程同生物材料的表面形貌密切相关, 其中材料表面形态对细胞骨架结构的影响也是调节干细胞向特定细胞谱系分化的最重要的因素之一[85]。研究证实相较于单一结构, 微纳米结构能够实现多级结构的协同调控, 共同促进组织再生: 一方面微米结构可以为成骨细胞系的生长、增殖、成骨分化、矿化和最终骨形成提供空间; 另一方面纳米结构可以调节成骨相关细胞的活性, 产生有利于成骨的微环境, 以增强成骨[11,86]。如前所述,微纳米结构可以通过多种方式促进组织再生, 包括诱导间充质干细胞的成骨分化, 抑制破骨细胞生成, 促进血管内皮细胞的血管分化以及参与骨免疫调节等(表1)。后续还需要更多的研究以深入阐明生物材料与骨缺损部位微环境之间的相互作用机制, 从而探索参与骨修复和重建的各种生物和物理微环境因素与生物材料之间的交互作用以及内在作用机制。
表1 微纳米结构生物材料用于成骨研究的文献总结
Table 1
| Material | Synthesis method | In vitro results | Animal model | In vivo results | Ref. |
|---|---|---|---|---|---|
| β-TCP scaffolds with micro/ nano surface topography | DLP printing and in situ growth crystal process | Promote osteogenic differentiation of stem cells | Rat skull defects | Improve the bone regeneration | [17] |
| Micro/nano-scale titania fiber-like network on the surface of Ti implants | One-step alkaline treatment in NaOH solution | Facilitate osteogenic and angiogenic differentiation of BMSCs and endothelial cells; Suppress M1 macrophages and stimulate M2 phenotype | Rabbit femur defects | Induce ameliorative osseointegration | [21] |
| MNBG/PLGA bi-layered membranes | Electrospinning | Promote osteogenesis | [24] | ||
| Micro-nano rough Ti6Al4V | Acid etch process | Improve osteogenic differentiation of MSCs | [26] | ||
| HA bioceramics with submicron- to nano- topographies | Sintering | Maintain the conformation of BMP-2, activate the osteogenic differentiation of BMSCs | Canine intramuscular implantation | Process excellent bone-like apatite forming ability and outstanding osteoinductivity | [28] |
| HA with micro/nano hierarchical structures | Photolithography and hydrothermal techniques | Promote osteogenic differentiation of hBMSCs and angiogenic acticvity of HUVECs | [41] | ||
| β-TCP/CaSiO3 composite ceramics with micro/ nano-HAp the surface layer | 3D bioplotting and hydrothermal treatment | Upregulate the cellular differentiation of mBMSCs and gene expression of HUVECs | Ectopic subcutaneous implantation at the back of rats | Promote capillary formation and bone augmentation | [45] |
| PEEK/CF/n-HA ternary biocomposite with micro/ nano-topographical surface | Oxygen plasma and sandblasting | Promote the proliferation and differentiation of MG-63 cells | Dog mandibles | Boost the osseointegration between implant and bone | [73] |
| Micro/nano structural silicon nitride and PEKK composite | Femtosecond laser ablation | Promote osteogenic differentiation of rBMSCs; Exhibit a greater bacteriostatic activity | Rabbit femur cavity defect | Promote osseointegration and bone repair | [75] |
| Silicate-based bioceramic with micro-nano surfaces and hollow channels | 3D printing and hydrothermal treatment | Facilitate the attachment and proliferation of BMSCs | Rabbit femur defects | Boost the newly bone formation | [80] |
| PLLA/CS composite scaffold with micro/nano- fiber hierarchical structure | 3D printing and thermally induced phase separation technology | Promote cell adhesion and proliferation | [87] |
另有研究指出微纳米结构能够赋予部分金属植入物如钛、钽、铪和锆及其氧化物抗菌性能, 从而实现细菌感染的局部治疗[88]。另外,由于聚合物基复合材料具有高拉伸强度、刚度、断裂韧性、耐磨性和耐腐蚀性, 进一步与微纳米结构生物活性陶瓷结合以模拟天然骨组织的结构和组成特性, 展现出良好的应用前景[89]。值得关注的是, 微纳米结构不仅能够增强生物活性陶瓷的生物学特性, 如骨诱导、骨整合和血管生成等, 还能够显著改善其机械性能, 为术后的骨组织再生系统提供足够的强度支持[45]。并且生物矿化材料如骨骼和牙齿等由于具有多尺度纳米结构和有序自组装特性, 展现出优异的机械性能如硬度、韧性、强度, 以及抗折性能[90-91]。且骨质疏松性骨晶体的结晶度低于正常骨[92]。受此启发, 在微纳米结构研究的基础上实现高结晶度且有序组装或能进一步提高生物活性陶瓷的力学性能和生物学性能。然而, 目前关于微纳米结构生物材料的研究还受到各种制备方法的限制, 合成出具有高生物活性和良好机械性能的生物材料仍有一定难度。另外, 深入了解微纳米结构生物材料在体内的降解速率和机制对于设计新的治疗方法也具有重要意义[93]。以上研究表明未来需要不断开发新技术以制备出具有从纳米尺度到宏观尺度的复杂层次结构且高度有序的新型骨组织工程生物材料以满足临床患者的需求。
参考文献
Applications of zeolitic imidazolate framework-8 (ZIF-8) in bone tissue engineering: a review
Functionalization of synthetic bone substitutes
SATB2: a versatile transcriptional regulator of craniofacial and skeleton development, neurogenesis and tumorigenesis, and its applications in regenerative medicine
Evaluation of rhBMP-2 and bone marrow derived stromal cell mediated bone regeneration using transgenic fluorescent protein reporter mice
Stromal cells and stem cells in clinical bone regeneration
Projection of osteoporosis-related fractures and costs in China: 2010-2050
A state-transition microsimulation model was used to project the substantial economic burden to the Chinese healthcare system of osteoporosis-related fractures. Annual number and costs of osteoporosis-related fractures were estimated to double by 2035 and will increase to 5.99 (95 % CI 5.44, 6.55) million fractures costing $25.43 (95 % CI 23.92, 26.95) billion by 2050. Consequently, cost-effective intervention policies must urgently be identified in an attempt to minimize the impact of fractures.The aim of the study was to project the osteoporosis-related fractures and costs for the Chinese population aged ≥50 years from 2010 to 2050.A state-transition microsimulation model was used to simulate the annual incident fractures and costs. The simulation was performed with a 1-year cycle length and from the Chinese healthcare system perspective. Incident fractures and annual costs were estimated from 100 unique patient populations for year 2010, by multiplying the age- and sex-specific annual fracture risks and costs of fracture by the corresponding population totals in each of the 100 categories. Projections for 2011-2050 were performed by multiplying the 2010 risks and costs of fracture by the respective annual population estimates. Costs were presented in 2013 US dollars.Approximately 2.33 (95 % CI 2.08, 2.58) million osteoporotic fractures were estimated to occur in 2010, costing $9.45 (95 % CI 8.78, 10.11) billion. Females sustained approximately three times more fractures than males, accounting for 76 % of the total costs from 1.85 (95 % CI 1.68, 2.01) million fractures. The annual number and costs of osteoporosis-related fractures were estimated to double by 2035 and will increase to 5.99 (95 % CI 5.44, 6.55) million fractures costing $25.43 (95 % CI 23.92, 26.95) billion by 2050.Our study demonstrated that osteoporosis-related fractures cause a substantial economic burden which will markedly increase over the coming decades. Consequently, healthcare resource planning must consider these increasing costs, and cost-effective screening and intervention policies must urgently be identified in an attempt to minimize the impact of fractures on the health of the burgeoning population as well as the healthcare budget.
Simple coating with fibronectin fragment enhances stainless steel screw osseointegration in healthy and osteoporotic rats
Metal implants are widely used to provide structural support and stability in current surgical treatments for bone fractures, spinal fusions, and joint arthroplasties as well as craniofacial and dental applications. Early implant-bone mechanical fixation is an important requirement for the successful performance of such implants. However, adequate osseointegration has been difficult to achieve especially in challenging disease states like osteoporosis due to reduced bone mass and strength. Here, we present a simple coating strategy based on passive adsorption of FN7-10, a recombinant fragment of human fibronectin encompassing the major cell adhesive, integrin-binding site, onto 316-grade stainless steel (SS). FN7-10 coating on SS surfaces promoted α5β1 integrin-dependent adhesion and osteogenic differentiation of human mesenchymal stem cells. FN7-10-coated SS screws increased bone-implant mechanical fixation compared to uncoated screws by 30% and 45% at 1 and 3 months, respectively, in healthy rats. Importantly, FN7-10 coating significantly enhanced bone-screw fixation by 57% and 32% at 1 and 3 months, respectively, and bone-implant ingrowth by 30% at 3 months compared to uncoated screws in osteoporotic rats. These coatings are easy to apply intra-operatively, even to implants with complex geometries and structures, facilitating the potential for rapid translation to clinical settings. Copyright © 2015 Elsevier Ltd. All rights reserved.
The relevance of biomaterials to the prevention and treatment of osteoporosis. opinion paper
Osteoporosis is a worldwide disease with a very high prevalence in humans older than 50. The main clinical consequences are bone fractures, which often lead to patient disability or even death. A number of commercial biomaterials are currently used to treat osteoporotic bone fractures, but most of these have not been specifically designed for that purpose. Many drug- or cell-loaded biomaterials have been proposed in research laboratories, but very few have received approval for commercial use. In order to analyze this scenario and propose alternatives to overcome it, the Spanish and European Network of Excellence for the Prevention and Treatment of Osteoporotic Fractures, "Ageing", was created. This network integrates three communities, e.g. clinicians, materials scientists and industrial advisors, tackling the same problem from three different points of view. Keeping in mind the premise "living longer, living better", this commentary is the result of the thoughts, proposals and conclusions obtained after one year working in the framework of this network. Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Monocyclic β-lactams loaded on hydroxyapatite: new biomaterials with enhanced antibacterial activity against resistant strains
The development of biomaterials able to act against a wide range of bacteria, including antibiotic resistant bacteria, is of great importance since bacterial colonization is one of the main causes of implant failure. In this work, we explored the possibility to functionalize hydroxyapatite (HA) nanocrystals with some monocyclic N-thio-substituted β-lactams. To this aim, a series of non-polar azetidinones have been synthesized and characterized. The amount of azetidinones loaded on HA could be properly controlled on changing the polarity of the loading solution and it can reach values up to 17 wt%. Data on cumulative release in aqueous solution show different trends which can be related to the lipophilicity of the molecules and can be modulated by suitable groups on the azetidinone. The examined β-lactams-HA composites display good antibacterial activity against reference Gram-positive and Gram-negative bacteria. However, the results of citotoxicity and antibacterial tests indicate that HA loaded with 4-acetoxy-1-(methylthio)-azetidin-2-one displays the best performance. In fact, this material strongly inhibited the bacterial growth of both methicillin resistant and methicillin susceptible clinical isolates of S. aureus from surgical bone biopsies, showing to be a very good candidate as a new functional biomaterial with enhanced antibacterial activity.
Microenvironment influences on human umbilical cord mesenchymal stem cell-based bone regeneration
Hierarchically designed bone scaffolds: from internal cues to external stimuli
The material and biological characteristics of osteoinductive calcium phosphate ceramics
The discovery of osteoinductivity of calcium phosphate (Ca-P) ceramics has set an enduring paradigm of conferring biological regenerative activity to materials with carefully designed structural characteristics. The unique phase composition and porous structural features of osteoinductive Ca-P ceramics allow it to interact with signaling molecules and extracellular matrices in the host system, creating a local environment conducive to new bone formation. Mounting evidence now indicate that the osteoinductive activity of Ca-P ceramics is linked to their physicochemical and three-dimensional structural properties. Inspired by this conceptual breakthrough, many laboratories have shown that other materials can be also enticed to join the rank of tissue-inducing biomaterials, and besides the bones, other tissues such as cartilage, nerves and blood vessels were also regenerated with the assistance of biomaterials. Here, we give a brief historical recount about the discovery of the osteoinductivity of Ca-P ceramics, summarize the underlying material factors and biological characteristics, and discuss the mechanism of osteoinduction concerning protein adsorption, and the interaction with different types of cells, and the involvement of the vascular and immune systems.
Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration
Tissue engineering is a promising strategy for bone regeneration in repairing massive bone defects. The surface morphology of implanted materials plays a key role in bone healing; these materials incorporate osteoinductive factors to improve the efficiency of bone regeneration.In the current study, nanostructured hydroxyapatite (nHAp) micro-spheres were prepared via a hydrothermal transformation method using calcium silicate (CS) microspheres as precursors; the CS microspheres were obtained by a spray-drying method. The nHAp microspheres constructed by the nano-whiskers significantly improved the ability of the microspheres to adsorb the bioactive protein (BMP-2) and reduce its initial burst release. To evaluate the in vivo bone regeneration of microspheres, both conventional hydroxyapatite (HAp) and nHAp microspheres were either loaded with recombinant human bone morphogenetic protein-2 (rhBMP-2) or not loaded with the protein; these microspheres were implanted in rat femoral bone defects for 4 and 8 weeks.The results of our three-dimensional (3D) micro-computed tomography (CT) and histomorphometric observations showed that the combination of the nano-structured surface and rhBMP-2 obviously improved osteogenesis compared to conventional HAp microspheres loaded with rhBMP-2. Our results suggest that the nHAp microspheres with a nanostructured surface adsorb rhBMP-2 for rapid bone formation; they therefore show the potential to act as carriers in bone tissue regeneration.
Important topics in the future of tissue engineering:comments from the participants of the 5th International Conference on Tissue Engineering at Kos, Greece
Stem cells: microenvironment, micro/nanotechnology, and application
Osteoporotic bone recovery by a bamboo-structured bioceramic with controlled release of hydroxyapatite nanoparticles
While most bone defects can be repaired spontaneously, the healing process can be complicated due to insufficient bone regeneration when osteoporosis occurs. Synthetic materials that intrinsically stimulate bone formation without inclusion of exogenous cells or growth factors represent a highly desirable alternative to current grafting strategies for the management of osteoporotic defects. Herein, we developed a series of hydroxyapatite bioceramics composed of a microwhiskered scaffold (wHA) reinforced with multiple layers of releasable hydroxyapatite nanoparticles (nHA). These novel bioceramics (nwHA) are tunable to optimize the loading amount of nHA for osteoporotic bone formation. The utility of nwHA bioceramics for the proliferation or differentiation of osteoporotic osteoblasts in vitro is demonstrated. A much more compelling response is seen when bioceramics are implanted in critical-sized femur defects in osteoporotic rats, as nwHA bioceramics promote significantly higher bone regeneration and delay adjacent bone loss. Moreover, the nwHA bioceramics loaded with a moderate amount of nHA can induce new bone formation with a higher degree of ossification and homogenization. Two types of osteogenesis inside the nwHA bioceramic pores were discovered for the first time, depending on the direction of growth of the new bone. The current study recommends that these tailored hybrid micro/nanostructured bioceramics represent promising candidates for osteoporotic bone repair.© 2022 The Authors.
Development of hierarchical porous bioceramic scaffolds with controlled micro/nano surface topography for accelerating bone regeneration
AMOT130/YAP pathway in topography-induced BMSC osteoblastic differentiation
Cell osteogenic bioactivity mediated precisely by varying scaled micro-pits on ordered micro/nano hierarchical structures of titanium
Osteoclast differentiation from human blood precursors on biomimetic calcium-phosphate substrates
The design of synthetic bone grafts to foster bone formation is a challenge in regenerative medicine. Understanding the interaction of bone substitutes with osteoclasts is essential, since osteoclasts not only drive a timely resorption of the biomaterial, but also trigger osteoblast activity. In this study, the adhesion and differentiation of human blood-derived osteoclast precursors (OCP) on two different micro-nanostructured biomimetic hydroxyapatite materials consisting in coarse (HA-C) and fine HA (HA-F) crystals, in comparison with sintered stoichiometric HA (sin-HA, reference material), were investigated. Osteoclasts were induced to differentiate by RANKL-containing supernatant using cell/substrate direct and indirect contact systems, and calcium (Ca) and phosphorus (P) in culture medium were measured. We observed that OCP adhered to the experimental surfaces, and that osteoclast-like cells formed at a rate influenced by the micro- and nano-structure of HA, which also modulate extracellular Ca. Qualitative differences were found between OCP on biomimetic HA-C and HA-F and their counterparts on plastic and sin-HA. On HA-C and HA-F cells shared typical features of mature osteoclasts, i.e. podosomes, multinuclearity, tartrate acid phosphatase (TRAP)-positive staining, and TRAP5b-enzyme release. However, cells were less in number compared to those on plastic or on sin-HA, and they did not express some specific osteoclast markers. In conclusion, blood-derived OCP are able to attach to biomimetic and sintered HA substrates, but their subsequent fusion and resorptive activity are hampered by surface micro-nano-structure. Indirect cultures suggest that fusion of OCP is sensitive to topography and to extracellular calcium.The novelty of the paper is the differentiation of human blood-derived osteoclast precursors, instead of mouse-derived macrophages as used in most studies, directly on biomimetic micro-nano structured HA-based surfaces, as triggered by osteoblast-produced factors (RANKL/OPG), and influenced by chemistry and topography of the substrate(s). Biomimetic HA-surfaces, like those obtained in calcium phosphate cements, are very different from the conventional calcium phosphate ceramics, both in terms of topography and ion exchange. The role of these factors in modulating precursors' differentiation and activity is analysed. The system is closely reproducing the physiological process of attachment of host cells and further maturation to osteoclasts toward resorption of the substrate, which occurs in vivo after filling bone defects with the calcium phosphate grafts.Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration
mTORC2 regulates hierarchical micro/nano topography-induced osteogenic differentiation via promoting cell adhesion and cytoskeletal polymerization
Different cell and tissue behavior of micro-/nano-tubes and micro-/nano-nets topographies on selective laser melting titanium to enhance osseointegration
Micro-/nano-tubes (TNTs) and micro-/nano-nets (TNNs) are the common and sensible choice in the first step of combined modifications of titanium surface for further functionalization in the purpose of extended indications and therapeutic effect. It is important to recognize the respective biologic reactions of these two substrates for guiding a biologically based first-step selection.TNTs were produced by anodic oxidation and TNNs were formed by alkali-heat treatment. The original selective laser melting (SLM) titanium surface was set as control. Surface characterization was evaluated by scanning electron microscopy, surface roughness, and water contact angle measurements. Osteoclastogenesis and osteogenesis were measured. MC3T3-E1 cells and RAW 264.7 cells were used for assay in terms of adhesion, proliferation, and differentiation. assessments were taken on Beagle dogs with micro-CT and histological analysis.TNN and TNT groups performed decreased roughness and increased hydrophilicity compared with SLM group. For biological detections, the highest ALP activity and osteogenesis-related genes expression were observed in TNT group followed by TNN group (P <0.05). Interestingly, when it comes to the osteoclastogenesis, TNNs displayed lowest TRAP activity and osteoclastogenesis-related genes expression and TNTs were lower than SLM but higher than TNNs (P <0.05). BV/TV around implants was highest in TNT group after 4 weeks (P <0.05). HE, ALP and TRAP staining showed that osteogenic and osteoclastic activity around TNTs were both higher than TNNs (P <0.05).TNNs and TNTs have dual advantages in promotion of osteogenesis and inhibition of osteoclastogenesis. Furthermore, TNNs showed better capability in inhibiting osteoclast activity while TNTs facilitated stronger osteogenesis. Our results implied that TNT substrates would take advantage in early application after implantation, while diseases with inappropriate osteoclast activity would prefer TNN substrates, which will guide a biologically based first-step selection on combined modification for different clinical purposes.© 2021 Yu et al.
A bi-layered membrane with micro-nano bioactive glass for guided bone regeneration
Involvement of Rac1 in the micro/nano-topography sensing and osteogenic differentiation in bone marrow mesenchymal stem cells
Human mesenchymal stem cell morphology, migration, and differentiation on micro and nano-textured titanium
Orthopedic implants rely on facilitating a robust interaction between the implant material surface and the surrounding bone tissue. Ideally, the interface will encourage osseointegration with the host bone, resulting in strong fixation and implant stability. However, implant failure can occur due to the lack of integration with bone tissue or bacterial infection. The chosen material and surface topography of orthopedic implants are key factors that influence the early events following implantation and may ultimately define the success of a device. Early attachment, rapid migration and improved differentiation of stem cells to osteoblasts are necessary to populate the surface of biomedical implants, potentially preventing biofilm formation and implant-associated infection. This article explores these early stem cell specific events by seeding human mesenchymal stem cells (MSCs) on four clinically relevant materials: polyether ether ketone (PEEK), Ti6Al4V (smooth Ti), macro-micro rough Ti6Al4V (Endoskeleton®), and macro-micro-nano rough Ti6Al4V (nanoLOCK®). The results demonstrate the incorporation of a hierarchical macro-micro-nano roughness on titanium produces a stellate morphology typical of mature osteoblasts/osteocytes, rapid and random migration, and improved osteogenic differentiation in seeded MSCs. Literature suggests rapid coverage of a surface by stem cells coupled with stimulation of bone differentiation minimizes the opportunity for biofilm formation while increasing the rate of device integration with the surrounding bone tissue..
Nanomaterial-based bone regeneration
Bone diseases/injuries have been driving an urgent quest for bone substitutes for bone regeneration. Nanoscaled materials with bone-mimicking characteristics may create suitable microenvironments to guide effective bone regeneration. In this review, the natural hierarchical architecture of bone and its regeneration mechanisms are elucidated. Recent progress in the development of nanomaterials which can promote bone regeneration through bone-healing mimicry (e.g., compositional, nanocrystal formation, structural, and growth factor-related mimicking) is summarized. The nanoeffects of nanomaterials on the regulation of bone-related biological functions are highlighted. How to prepare nanomaterials with combinative bone-biomimicry features according to the bone healing process is prospected in order to achieve rapid bone regeneration in situ.
Design of hydroxyapatite bioceramics with micro-/nano-topographies to regulate the osteogenic activities of bone morphogenetic protein-2 and bone marrow stromal cells
Biomimicking the nanostructure of natural bone apatite to enhance the bioactivity of hydroxyapatite (HA) biomaterials is an eternal topic in the bone regeneration field. In the present study, we designed four kinds of HA bioceramics with micro- to nanosized grains and investigated the effects of bioceramic topographies on the structures of bone morphogenetic protein-2 (BMP-2) and the effects on the responses of bone marrow stromal cells (BMSCs). Compared to the samples with submicron-scale crystalline particles, HA bioceramics with grain sizes of 104.6 ± 27.8 nm exhibited increased roughness, improved hydrophilicity and enhanced mechanical properties. The synergistic effects of these surface characteristics could well maintain the conformation of BMP-2, facilitate cell adhesion and spreading, and activate the osteogenic differentiation of BMSCs. Furthermore, SBF immersion and in vivo canine intramuscular implantation confirmed that the HA bioceramics with nanotopography also processed excellent bone-like apatite forming ability and outstanding osteoinductivity. In summary, these findings suggest that the nanotopography of HA bioceramics is a critical factor to enhance their bioactivity and osteoinductivity.
Enhanced bone regenerative properties of calcium phosphate ceramic granules in rabbit posterolateral spinal fusion through a reduction of grain size
Osteoinductivity is a crucial factor to determine the success and efficiency of posterolateral spinal fusion (PLF) by employing calcium phosphate (Ca-P) bioceramics. In this study, three kinds of Ca-P ceramics with microscale to nanoscale gain size (BCP-control, BCP-micro and BCP-nano) were prepared and their physicochemical properties were characterized. BCP-nano had the spherical shape and nanoscale gain size, BCP-micro had the spherical shape and microscale gain size, and BCP-control (BAM®) had the irregular shape and microscale gain size. The obtained BCP-nano with specific nanotopography could well regulate protein adsorption and osteogenic differentiation of MC3T3 cells. rabbit PLF procedures further confirmed that nanotopography of BCP-nano might be responsible for the stronger bone regenerative ability comparing with BCP-micro and BCP-control. Collectedly, due to nanocrystal similarity with natural bone apatite, BCP-nano has excellent efficacy in guiding bone regeneration of PLF, and holds great potentials to become an alternative to standard bone grafts for future clinical applications.© 2021 The Authors.
The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells
The micro/nano hybrid structure is considered to be a biomaterial characteristic to stimulate osteogenesis by mimicking the three-dimensional structure of the bone matrix. However, the mechanism of the hybrid structure induced osteogenic differentiation of stem cells is still unknown. For elucidating the mechanisms, one of the challenge is to directly fabricate micro/nano hybrid structure on bioceramics because of its brittleness. In this study, hydroxyapatite (HA) bioceramics with the micro/nano hybrid structure were firstly fabricated via a hydrothermal treatment and template method, and the effect of the different surface structures on the expression of integrins, BMP2 signaling pathways and cell-cell communication was investigated. Interestingly, the results suggested that the osteogenic differentiation induced by micro/nano structures was modulated first through activating integrins and then further activating BMP2 signaling pathway and cell-cell communication, while activated BMP2 could in turn activate integrins and Cx43-related cell-cell communication. Furthermore, differences in activation of integrins, BMP2 signaling pathway, and gap junction-mediated cell-cell communication were observed, in which nanorod and micropattern structures activated different integrin subunits, BMP downstream receptors and Cx43. This finding may explain the synergistic effect of the micro/nano hybrid structure on the activation of osteogenic differentiation of BMSCs. Based on our study, we concluded that the different activation mechanisms of micro- and nano-structures led to the synergistic stimulatory effect on integrin activation and osteogenesis, in which not only the direct contact of cells on micro/nano structure played an important role, but also other surface characteristics such as protein adsorption might contribute to the bioactive effect.The micro/nano hybrid structure has been found to have synergistic bioactivity on osteogenesis. However, it is still a challenge to fabricate the hybrid structure directly on the bioceramics, and the role of micro- and nano-structure, in particular the mechanism of the micro/nano-hybrid structure induced stem cell differentiation is still unknown. In this study, we firstly fabricated hydroxyapatite bioceramics with the micro/nano hybrid structure, and then investigated the effect of different surface structure on expression of integrins, BMP2 signaling pathways and cell-cell communication. Interestingly, we found that the osteogenic differentiation induced by structure was modulated first through activating integrins and then further activating BMP2 signaling pathway and cell-cell communication, and activated BMP2 could in turn activate some integrin subunits and Cx43-related cell-cell communication. Furthermore, differences in activation of integrins, BMP2 signaling pathway, and gap junction-mediated cell-cell communication were observed, in which nanorod and micropattern structures activated different integrin subunits, BMP downstream receptors and Cx43. This finding may explain the synergistic effect of the micro/nano hybrid structure on the activation of osteogenic differentiation of BMSCs. Based on our study, we concluded that the different activation mechanisms of micro- and nano-structures led to the synergistic stimulatory effect on integrin activation and osteogenesis, in which not only the direct contact of cells on micro/nano structure played an important role, but also other surface characteristics such as protein adsorption might contribute to the bioactive effect.Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells
Tissue engineering strategies to construct vascularized bone grafts potentially revolutionize the treatment of massive bone loss. The surface topography of the grafts plays critical roles on bone regeneration, while adipose derived stem cells (ASCs) are known for their capability to promote osteogenesis and angiogenesis when applied to bone defects. In the present study, the effects of hydroxyapatite (HAp) bioceramic scaffolds with nanosheet, nanorod, and micro-nano-hybrid (the hybrid of nanorod and microrod) surface topographies on attachment, proliferation and osteogenic differentiation, as well as the expression of angiogenic factors of rat ASCs were systematically investigated. The results showed that the HAp bioceramic scaffolds with the micro-/nano-topography surfaces significantly enhanced cell attachment and viability, alkaline phosphatase (ALP) activity, and mRNA expression levels of osteogenic markers and angiogenic factors of ASCs. More importantly, the biomimetic feature of the hierarchical micro-nano-hybrid surface topography showed the highest stimulatory effect. The activation in Akt signaling pathway was observed in ASCs cultured on HAp bioceramics with nanorod, and micro-nano-hybrid surface topographies. Moreover, these induction effects could be repressed by Akt signaling pathway inhibitor LY294002. Finally, the in vivo bone regeneration results of rat critical-sized calvarial defect models confirmed that the combination of the micro-nano-hybrid surface and ASCs could significantly enhance both osteogenesis and angiogenesis as compared with the control HAp bioceramic scaffold with traditional smooth surface. Our results suggest that HAp bioceramic scaffolds with micro-nano-hybrid surface can act as cell carrier for ASCs, and consequently combine with ASCs to construct vascularized tissue-engineered bone.Copyright © 2014 Elsevier Ltd. All rights reserved.
Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs
Healing of osteoporotic bone defects by micro-/nano-structured calcium phosphate bioceramics
It is a particularly critical challenge to achieve enough bone regeneration in osteoporotic fractures with bone defects. In the present study, we designed a micro-/nano-structured calcium phosphate bioceramic composed of a nanoparticle-reinforced micro-whisker backbone (nwCaP). This sandwich-structured bioceramic exhibited a higher compressive strength, a suitable degradation rate and better cell attachment than traditional or intermediate bioceramics. In a rat model of osteoporotic bone defects, the nwCaP group showed a reduced fracture occurrence and an effective new bone substitution rate, as characterized by micro-CT analysis. The increased bone formation rate and greater amount of new bone formed within the defected area of the nwCaP group was revealed by the serum PINP level and histological staining. Moreover, a gene microarray study indicated that the promotion of osteogenesis might be attributed to selectively upregulated fibroblast growth factor 23 (FGF23) expression in cells co-cultured with the nwCaP bioceramic. Furthermore, the JAK2 signal pathway was confirmed to be involved in the nwCaP-induced elevation of FGF23 expression using primary osteoblasts derived from osteoporotic rats. Collectively, the findings suggested that the micro-/nano-structured bioceramic could enhance osteoporotic bone regeneration and presents a promising strategy for healing bone defects in osteoporosis.
The role of anthrax toxin protein receptor 1 as a new mechanosensor molecule and its mechanotransduction in BMSCs under hydrostatic pressure
Anthrax toxin protein receptor (ANTXR) 1 has many similarities to integrin and is regarded in some respects as a single-stranded integrin protein. However, it is not clear whether ANTXR1 responds to mechanical signals secondary to the activation of integrins or whether it is a completely new, independent and previously undiscovered mechanosensor that responds to an undefined subset of mechanical signaling molecules. Our study demonstrates that ANTXR1 is a novel mechanosensor on the cell membrane, acting independently from the classical mechanoreceptor molecule integrinβ1. We show that bone marrow stromal cells (BMSCs) respond to the hydrostatic pressure towards chondrogenic differentiation partly through the glycogen synthase kinase (GSK) 3β/β-Catenin signaling pathway, which can be partly regulated by ANTXR1 and might be related to the direct binding between ANTXR1 and low-density lipoprotein receptor-related protein (LRP) 5/6. In addition, ANTXR1 specifically activates Smad2 and upregulates Smad4 expression to facilitate the transport of activated Smad2 to the nucleus to regulate chondrogenesis, which might be related to the direct binding between ANTXR1 and Actin/Fascin1. We also demonstrate that ANTXR1 binds to some extent with integrinβ1, but this interaction does not affect the expression and function of either protein under pressure. Thus, we conclude that ANTXR1 plays a crucial role in BMSC mechanotransduction and controls specific signaling pathways that are distinct from those of integrin to influence the chondrogenic responses of BMSCs under hydrostatic pressure.
Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation
Drug-loaded biomimetic ceramics for tissue engineering
The mimesis of biological systems has been demonstrated to be an adequate approach to obtain tissue engineering scaffolds able to promote cell attachment, proliferation, and differentiation abilities similar to those of autologous tissues. Bioceramics are commonly used for this purpose due to their similarities to the mineral component of hard tissues as bone. Furthermore, biomimetic scaffolds are frequently loaded with diverse therapeutic molecules to enhance their biological performance, leading to final products with advanced functionalities. In this review, we aim to describe the already developed bioceramic-based biomimetic systems for drug loading and local controlled release. We will discuss the mechanisms used for the inclusion of therapeutic molecules on the designed systems, paying special attention to the identification of critical parameters that modulate drug loading and release kinetics on these scaffolds.
Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin β1/FAKpY397/MAPK pathway
Effects of hydroxyapatite surface nano/micro-structure on osteoclast formation and activity
A novel magnesium ion- incorporating dual-crosslinked hydrogel to improve bone scaffold- mediated osteogenesis and angiogenesis
Pharmacological manipulation of macrophage autophagy effectively rejuvenates the regenerative potential of biodegrading vascular graft in aging body
Declined regenerative potential and aggravated inflammation upon aging create an inappropriate environment for arterial regeneration. Macrophages are one of vital effector cells in the immune microenvironment, especially during biomaterials mediated repairing process. Here, we revealed that the macrophage autophagy decreased with aging, which led to aggravated inflammation, thereby causing poor vascular remodeling of artificial grafts in aging body. Through loading the autophagy-targeted drugs, rapamycin and 3-MA (3-methyladenine), in PCL (polycaprolactone) sheath of the PGS (poly glycerol sebacate) - PCL vascular graft, the essential role of macrophage autophagy was confirmed in regulating macrophage polarization and biomaterial degradation. Moreover, the utilization of rapamycin promoted anti-inflammatory polarization of macrophage by activating autophagy, which further promoted myogenic differentiation of vascular progenitor cells and accelerated endothelialization. Our study elucidated the contribution of pharmacological manipulation of macrophage autophagy in promoting regeneration of small caliber artery, which may pave a new avenue for clinical translation of vascular grafts in aging body.© 2021 The Authors.
Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation
Insights into the angiogenic effects of nanomaterials: mechanisms involved and potential applications
The vascular system, which transports oxygen and nutrients, plays an important role in wound healing, cardiovascular disease treatment and bone tissue engineering. Angiogenesis is a complex and delicate regulatory process. Vascular cells, the extracellular matrix (ECM) and angiogenic factors are indispensable in the promotion of lumen formation and vascular maturation to support blood flow. However, the addition of growth factors or proteins involved in proangiogenic effects is not effective for regulating angiogenesis in different microenvironments. The construction of biomaterial scaffolds to achieve optimal growth conditions and earlier vascularization is undoubtedly one of the most important considerations and major challenges among engineering strategies. Nanomaterials have attracted much attention in biomedical applications due to their structure and unique photoelectric and catalytic properties. Nanomaterials not only serve as carriers that effectively deliver factors such as angiogenesis-related proteins and mRNA but also simulate the nano-topological structure of the primary ECM of blood vessels and stimulate the gene expression of angiogenic effects facilitating angiogenesis. Therefore, the introduction of nanomaterials to promote angiogenesis is a great helpful to the success of tissue regeneration and some ischaemic diseases. This review focuses on the angiogenic effects of nanoscaffolds in different types of tissue regeneration and discusses the influencing factors as well as possible related mechanisms of nanomaterials in endothelial neovascularization. It contributes novel insights into the design and development of novel nanomaterials for vascularization and therapeutic applications.
Micro/nano-net guides M2-pattern macrophage cytoskeleton distribution via Src-ROCK signalling for enhanced angiogenesis
Micro-nano bioactive glass particles incorporated porous scaffold for promoting osteogenesis and angiogenesis in vitro
3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo
The role of autophagy in the process of osseointegration around titanium implants with micro- nano topography promoted by osteoimmunity
Osteoimmunity plays an important role in the process of implant osseointegration. Autophagy is a conservative metabolic pathway of eukaryotic cells, but whether the interaction between autophagy and osteoimmunity plays a key role in osseointegration remains unclear. In this study, we prepared smooth titanium disks and micro-nano topography titanium disks, to study the immune microenvironment of RAW264.7 cells, and prepared the conditioned medium to study the effect of immune microenvironment on the osteogenesis and autophagy of MC3T3-E1 cells. Autophagy inhibitor 3-MA was used to inhibit autophagy to observe the change of expression of osteogenic markers. The results showed that the micro-nano topography titanium disks could stimulate RAW264.7 cells to differentiate into M2 type, forming an anti-inflammatory immune microenvironment; compared with the control group, the anti-inflammatory immune microenvironment promoted the proliferation and differentiation of osteoblasts better. The anti-inflammatory immune environment activated the autophagy level of osteoblasts, while the expression of osteogenic markers was down-regulated after inhibition of autophagy. These results indicate that anti-inflammatory immune microenvironment can promote cell proliferation and osteogenic differentiation, autophagy plays an important role in this process. This study further explains the mechanism of implant osseointegration in osteoimmune microenvironment, and provides reference for improving implant osseointegration.
Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration
Immune cells play vital roles in regulating bone dynamics. Successful bone regeneration requires a favourable osteo-immune environment. The high plasticity and diversity of immune cells make it possible to manipulate the osteo-immune response of immune cells, thus modulating the osteoimmune environment and regulating bone regeneration. With the advancement in nanotechnology, nanotopographies with different controlled surface properties can be fabricated. On tuning the surface properties, the osteo-immune response can be precisely modulated. This highly tunable characteristic and immunomodulatory effects make nanotopography a promising strategy to precisely manipulate osteoimmunomdulation for bone tissue engineering applications. This review first summarises the effects of the immune response during bone healing to show the importance of regulating the immune response for the bone response. The plasticity of immune cells is then reviewed to provide rationales for manipulation of the osteoimmune response. Subsequently, we highlight the current types of nanotopographies applied in bone biomaterials and their fabrication techniques, and explain how these nanotopographies modulate the immune response and the possible underlying mechanisms. The effects of immune cells on nanotopography-mediated osteogenesis are emphasized, and we propose the concept of "nano-osteoimmunomodulation" to provide a valuable strategy for the development of nanotopographies with osteoimmunomodulatory properties that can precisely regulate bone dynamics.
Nanoporosity of alumina surfaces induces different patterns of activation in adhering monocytes/macrophages
Effects of nanoporous alumina on inflammatory cell response
The present study focuses on the effects of nanoscale porosity on inflammatory response in vitro and in vivo. Nanoporous alumina membranes with different pore sizes, 20 and 200 nm in diameter, were used. We first evaluated cell/alumina interactions in vitro by observing adhesion, proliferation, and activation of a murine fibroblast and a macrophage cell line. To investigate the chronic inflammatory response, the membranes were implanted subcutaneously in mice for 2 weeks. Cell recruitment to the site of implantation was determined by histology and the production of cytokines was measured by protein array analysis. Both in vitro and in vivo studies showed that 200 nm pores induced a stronger inflammatory response as compared to the alumina with 20 nm pores. This was observed by an increase in macrophage activation in vitro as well as higher cell recruitment and generation of proinflammatory cytokines around the alumina with 200 nm pores, in vivo. Our results suggest that nanofeatures can be modulated in order to control the inflammatory response to implants.© 2013 Wiley Periodicals, Inc.
Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages
Comparison of the osteogenic capability of rat bone mesenchymal stem cells on collagen, collagen/hydroxyapatite, hydroxyapatite and biphasic calcium phosphate
Collagen (COL), collagen/hydroxyapatite (COL/HA), HA and biphasic calcium phosphate were prepared as representative bone grafting materials with composition analogous to bone, and their structural characteristics were analyzed. The rat bone mesenchymal stem cells (BMSCs) were further seeded onto four groups of materials, and BMSCs grown in basic medium and standard osteogenic medium were set as controls of a reference model to show the basic and osteogenic behavior of cells without the intervention of materials. Cellular behaviors were characterized, including proliferation, spreading morphology and expression of osteogenesis factors. The rat BMSCs proliferated properly with time on four groups of materials as well on two groups of controls, and typical cuboidal, polygonal and extremely-elongated morphologies of cells were observed. According to the real-time polymerase chain reaction data, a higher osteogenic gene expression level was dependent upon the growing morphology but not the proliferation rate of cells, and the osteogenic differentiation capacity of cells onto four groups of materials varied in specific genes. In general, BMSCs exhibited the highest osteogenic capacity onto COL/HA, but the poorest onto HA. The growing behaviors of cells on materials were further discussed in comparison with the cases of OC and BC of the reference model. The present attempt to comparatively analyze cell experimental data with a reference model is expected to be useful for revealing the difference in the osteogenic capability of MSCs onto materials or even the bioactivity of materials.
Polyetheretherketone and its composites for bone replacement and regeneration
In this article, recent advances in the development, preparation, biocompatibility and mechanical properties of polyetheretherketone (PEEK) and its composites for hard and soft tissue engineering are reviewed. PEEK has been widely employed for fabricating spinal fusions due to its radiolucency, chemical stability and superior sterilization resistance at high temperatures. PEEK can also be tailored into patient-specific implants for treating orbital and craniofacial defects in combination with additive manufacturing process. However, PEEK is bioinert, lacking osseointegration after implantation. Accordingly, several approaches including surface roughening, thin film coating technology, and addition of bioactive hydroxyapatite (HA) micro-/nanofillers have been adopted to improve osseointegration performance. The elastic modulus of PEEK is 3.7–4.0 GPa, being considerably lower than that of human cortical bone ranging from 7–30 GPa. Thus, PEEK is not stiff enough to sustain applied stress in load-bearing orthopedic implants. Therefore, HA micro-/nanofillers, continuous and discontinuous carbon fibers are incorporated into PEEK for enhancing its stiffness for load-bearing applications. Among these, carbon fibers are more effective than HA micro-/nanofillers in providing additional stiffness and load-bearing capabilities. In particular, the tensile properties of PEEK composite with 30wt% short carbon fibers resemble those of cortical bone. Hydrophobic PEEK shows no degradation behavior, thus hampering its use for making porous bone scaffolds. PEEK can be blended with hydrophilic polymers such as polyglycolic acid and polyvinyl alcohol to produce biodegradable scaffolds for bone tissue engineering applications.
Bioengineered living bone grafts-a concise review on bioreactors and production techniques in vitro
It has been observed that bone fractures carry a risk of high mortality and morbidity. The deployment of a proper bone healing method is essential to achieve the desired success. Over the years, bone tissue engineering (BTE) has appeared to be a very promising approach aimed at restoring bone defects. The main role of the BTE is to apply new, efficient, and functional bone regeneration therapy via a combination of bone scaffolds with cells and/or healing promotive factors (e.g., growth factors and bioactive agents). The modern approach involves also the production of living bone grafts in vitro by long-term culture of cell-seeded biomaterials, often with the use of bioreactors. This review presents the most recent findings concerning biomaterials, cells, and techniques used for the production of living bone grafts under in vitro conditions. Particular attention has been given to features of known bioreactor systems currently used in BTE: perfusion bioreactors, rotating bioreactors, and spinner flask bioreactors. Although bioreactor systems are still characterized by some limitations, they are excellent platforms to form bioengineered living bone grafts in vitro for bone fracture regeneration. Moreover, the review article also describes the types of biomaterials and sources of cells that can be used in BTE as well as the role of three-dimensional bioprinting and pulsed electromagnetic fields in both bone healing and BTE.
Poloxamer-based scaffolds for tissue engineering applications: a review
Poloxamer is a triblock copolymer with amphiphilicity and reversible thermal responsiveness and has wide application prospects in biomedical applications owing to its multifunctional properties. Poloxamer hydrogels play a crucial role in the field of tissue engineering and have been regarded as injectable scaffolds for loading cells or growth factors (GFs) in the last few years. Hydrogel micelles can maintain the integrity and stability of cells and GFs and form an appropriate vascular network at the application site, thus creating an appropriate microenvironment for cell growth, nerve growth, or bone integration. The injectability and low toxicity of poloxamer hydrogels make them a noninvasive method. In addition, they can also be good candidates for bio-inks, the raw material for three-dimensional (3D) printing. However, the potential of poloxamer hydrogels has not been fully explored owing to the complex biological challenges. In this review, the latest progress and cutting-edge research of poloxamer-based scaffolds in different fields of application such as the bone, vascular, cartilage, skin, nervous system, and organs in tissue engineering and 3D printing are reviewed, and the important roles of poloxamers in tissue engineering scaffolds are discussed in depth.
Biodegradable materials for bone defect repair
Applications of ceramic/graphene composites and hybrids
Research activity on ceramic/graphene composites and hybrids has increased dramatically in the last decade. In this review, we provide an overview of recent contributions involving ceramics, graphene, and graphene-related materials (GRM, i.e., graphene oxide, reduced graphene oxide, and graphene nanoplatelets) with a primary focus on applications. We have adopted a broad scope of the term ceramics, therefore including some applications of GRM with certain metal oxides and cement-based matrices in the review. Applications of ceramic/graphene hybrids and composites cover many different areas, in particular, energy production and storage (batteries, supercapacitors, solar and fuel cells), energy harvesting, sensors and biosensors, electromagnetic interference shielding, biomaterials, thermal management (heat dissipation and heat conduction functions), engineering components, catalysts, etc. A section on ceramic/GRM composites processed by additive manufacturing methods is included due to their industrial potential and waste reduction capability. All these applications of ceramic/graphene composites and hybrids are listed and mentioned in the present review, ending with the authors’ outlook of those that seem most promising, based on the research efforts carried out in this field.
An overview of graphene-based hydroxyapatite composites for orthopedic applications
Hydroxyapatite (HA) is an attractive bioceramic for hard tissue repair and regeneration due to its physicochemical similarities to natural apatite. However, its low fracture toughness, poor tensile strength and weak wear resistance become major obstacles for potential clinical applications. One promising method to tackle with these problems is exploiting graphene and its derivatives (graphene oxide and reduced graphene oxide) as nanoscale reinforcement fillers to fabricate graphene-based hydroxyapatite composites in the form of powders, coatings and scaffolds. The last few years witnessed increasing numbers of studies on the preparation, mechanical and biological evaluations of these novel materials. Herein, various preparation techniques, mechanical behaviors and toughen mechanism, the in vivo biocompatible analysis, antibacterial properties of the graphene-based HA composites are presented in this review.
Bone response to laser-induced micro- and nano-size titanium surface features
This study explored whether laser-induced, site-specific implant surface modifications with micro- and nano-scale topography were able to promote bone formation. The aim was to evaluate the biomechanical and histological response to partly laser-modified titanium implants in comparison with machined implants. After an early 8-week healing period in rabbit tibia and femur, a 250% increase in removal torque was demonstrated for the partly laser-modified surface. Further, different fracture mechanisms were demonstrated for the two surfaces. Histologically, significantly more bone was found in direct contact with the laser-modified surface for the implants in the tibia sites, and a similar amount of bone tissue was observed in contact with the implant in the femoral sites. In conclusion, an improved bone-implant interface anchorage was promoted by an increase in micro- and nano-scale implant surface topography and surface oxide induced by topological laser treatment.Nanosized grooves in titanium implants markedly improve bone-implant anchorage by increasing the amount of bone formed in direct contact with the metal prosthesis.Copyright © 2011 Elsevier Inc. All rights reserved.
Comparative study on 3D printed Ti6Al4V scaffolds with surface modifications using hydrothermal treatment and microarc oxidation to enhance osteogenic activity
Titanium (Ti) and its alloys have been widely used in clinics as preferred materials for bone tissue repair and replacement. However, the lack of biological activity of Ti limits its clinical applications. Surface modification of Ti with bioactive elements has always been a research hotspot. In this study, to promote the osseointegration of Ti6Al4V (Ti64) implants, calcium (Ca), oxygen (O), and phosphorus (P) codoped multifunctional micro-nanohybrid coatings were prepared on a three-dimensional (3D) printed porous Ti64 surface by microarc oxidation (MAO) and a hydrothermal method (HT). The surface morphologies, chemical compositions, and surface/cell interactions of the obtained coatings were studied. experiments indicated that all hybrid coating-modified Ti64 implants could enhance protein adsorption and MC3T3 osteoblasts' activity, adhesion, and differentiation ability. experiments showed that the hybrid coating promoted early osseointegration. By comparison, microarc oxidation-treated Ti64 (M-Ti) has the best biological activity and the strongest ability of osseointegration. It provides important theoretical significance and potential application prospects for improving the biological activity of Ti implants.© 2021 The Authors. Published by American Chemical Society.
The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions
Hierarchical hybrid micro/nano-textured titanium surface topographies with titania nanotubes were produced by simple acid etching followed by anodization to mimic the hierarchical structure of bone tissues. Primary rat osteoblasts were used to evaluate the bioactivity. The microtopography formed by acid etching of titanium induced inconsistent osteoblast functions with initial cell adhesion and osteogenesis-related gene expression being dramatically enhanced while other cell behaviors such as proliferation, intracellular total protein synthesis and alkaline phosphatase activity, collagen secretion, and extracellular matrix mineralization being depressed. In comparison, addition of nanotubes to the microtopography led to enhancement of multiple osteoblast functions. Nearly all the cell functions investigated in this study were retained or promoted. Compared to a microtopography, the enhancement of multiple cell functions observed from the hierarchical micro/nano-textured surfaces is expected to lead to faster bone maturation around the titanium implants without compromising the bone mass. In addition, the hierarchical micro/nano-textured surfaces still retain the mechanical interlocking ability of the microtopography thereby boding well for osseointegration. Our study reveals a synergistic role played by the micro and nanotopographies in osteoblast functions and provides insight to the design of better biomedical implant surfaces.Copyright 2010 Elsevier Ltd. All rights reserved.
Enhanced bone- integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy
Micro-/nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization
Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity
The effects of Sr-incorporated micro/nano rough titanium surface on rBMSC migration and osteogenic differentiation for rapid osteointegration
Recruitment of endogenous bone marrow-derived mesenchymal stem cells (BMSCs) has been widely discussed as an alternative strategy for bone regeneration. Strontium (Sr) is known to direct the BMSCs' commitment to the bone lineage and encourage bone formation; however, the underlying mechanisms remain elusive. In this study, an Sr-incorporated micro/nano rough titanium surface (MNT-Sr) was fabricated by hydrothermal treatment in an attempt to facilitate BMSCs' recruitment and their osteogenic differentiation to enhance rapid osseointegration. Micro rough titanium (MT) was set as the control biomaterial. In vitro, MNT-Sr and its extracts promoted the migration and osteogenic differentiation of BMSCs. In animal studies, green fluorescent protein (GFP)-labeled BMSCs were intravenously injected into wild-type rats for tracing before tibial implantation surgery. The GFP+BMSC recruitment to the implantation site was successfully triggered by MNT-Sr implantation. A trend for increased bone area (BA%), bone-implant contact (BIC%) and removal torque values (RTVs) was observed for the MNT-Sr implant compared to that observed for MT at 2 weeks. Advanced mechanism analysis indicated that Sr2+ enhanced the SDF-1α/CXCR4 signaling pathway both in vitro and in vivo. Taken together, these findings suggest that MNT-Sr has promising therapeutic potential for future use in dental implants by homing endogenous stem cells to stimulate bone regeneration.
Bioinspired micro/nano fabrication on dental implant-bone interface
Micro/nano structural tantalum coating for enhanced osteogenic differentiation of human bone marrow stem cells
An investigation of the mineral in ductile and brittle cortical mouse bone
Polymer-based scaffolds for soft-tissue engineering
Biomaterials have been used since ancient times. However, it was not until the late 1960s when their development prospered, increasing the research on them. In recent years, the study of biomaterials has focused mainly on tissue regeneration, requiring a biomaterial that can support cells during their growth and fulfill the function of the replaced tissue until its regeneration. These materials, called scaffolds, have been developed with a wide variety of materials and processes, with the polymer ones being the most advanced. For this reason, the need arises for a review that compiles the techniques most used in the development of polymer-based scaffolds. This review has focused on three of the most used techniques: freeze-drying, electrospinning and 3D printing, focusing on current and future trends. In addition, the advantages and disadvantages of each of them have been compared.
Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering
Presently the majority of tissue engineering approaches aimed at regenerating bone relies only on post-implantation vascularization. Strategies that include seeding endothelial cells (ECs) on biomaterials and promoting their adhesion, migration and functionality might be a solution for the formation of vascularized bone. Nano/micro-fiber-combined scaffolds have an innovative structure, inspired by extracellular matrix (ECM) that combines a nano-network, aimed to promote cell adhesion, with a micro-fiber mesh that provides the mechanical support. In this work we addressed the influence of this nano-network on growth pattern, morphology, inflammatory expression profile, expression of structural proteins, homotypic interactions and angiogenic potential of human EC cultured on a scaffold made of a blend of starch and poly(caprolactone). The nano-network allowed cells to span between individual micro-fibers and influenced cell morphology. Furthermore, on nano-fibers as well as on micro-fibers ECs maintained the physiological expression pattern of the structural protein vimentin and PECAM-1 between adjacent cells. In addition, ECs growing on the nano/micro-fiber-combined scaffold were sensitive to pro-inflammatory stimulus. Under pro-angiogenic conditions in vitro, the ECM-like nano-network provided the structural and organizational stability for ECs' migration and organization into capillary-like structures. The architecture of nano/micro-fiber-combined scaffolds elicited and guided the 3D distribution of ECs without compromising the structural requirements for bone regeneration.
Micro/nano multilayered scaffolds of plga and collagen by alternately electrospinning for bone tissue engineering
The dual extrusion electrospinning technique was used to fabricate multilayered 3D scaffolds by stacking microfibrous meshes of poly(lactic acid-co-glycolic acid) (PLGA) in alternate fashion to micro/nano mixed fibrous meshes of PLGA and collagen. To fabricate the multilayered scaffold, 35 wt% solution of PLGA in THF-DMF binary solvent (3:1) and 5 wt% solution of collagen in hexafluoroisopropanol (HFIP) with and without hydroxyapatite nanorods (nHA) were used. The dual and individual electrospinning of PLGA and collagen were carried out at flow rates of 1.0 and 0.5 mL/h, respectively, at an applied voltage of 20 kV. The density of collagen fibers in multilayered scaffolds has controlled the adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells. The homogeneous dispersion of glutamic acid-modified hydroxyapatite nanorods (nHA-GA) in collagen solution has improved the osteogenic properties of fabricated multilayered scaffolds. The fabricated multilayered scaffolds were characterized using FT-IR, X-ray photoelectron spectroscopy, and transmission electron microscopy (TEM). The scanning electron microscopy (FE-SEM) was used to evaluate the adhesion and spreads of MC3T3-E1 cells on multilayered scaffolds. The activity of MC3T3-E1 cells on the multilayered scaffolds was evaluated by applying MTT, alkaline phosphatase, Alizarin Red, von Kossa, and cytoskeleton F-actin assaying protocols. The micro/nano fibrous PLGA-Col-HA scaffolds were found to be highly bioactive in comparison to pristine microfibrous PLGA and micro/nano mixed fibrous PLGA and Col scaffolds.
Core-sheath micro/ nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications
A novel nano/micro-fibrous scaffold by melt-spinning method for bone tissue engineering
Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone- nanohydroxyapatite biocomposite
3D printed dual-functional biomaterial with self-assembly micro-nano surface and enriched nano argentum for antibacterial and bone regeneration
Enhanced bacteriostatic activity, osteogenesis and osseointegration of silicon nitride/ polyetherketoneketone composites with femtosecond laser induced micro/ nano structural surface
Tailoring the nanostructured surfaces of hydroxyapatite bioceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation
Preparation of micro/nano- structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration
The surface microstructures of calcium phosphate ceramics play an essential role in determining bone regeneration. However, it is difficult to produce micro/nano-structures on the surface of the porous hydroxyapatite (HA) scaffolds. In this study, we successfully developed and fabricated various micro/nano-structured surfaces on the HA scaffolds in copper ion (Cu2+)-containing solutions under hydrothermal conditions. The micro/nano-structures on the surface of the HA scaffolds were controlled by modulating the Cu2+ concentrations during the hydrothermal process. With an increase in the Cu2+ concentration, the surface morphology of the HA scaffolds changed significantly from sphere-like to flower-like, before becoming nano-structures. These findings indicated that the Cu2+ concentration affects the morphologies of calcium phosphate coatings that grow on the HA scaffolds. In vitro endothelial cell (EC) cultures showed that the cell proliferation was significantly enhanced when cultured on the flower-like morphology compared with other morphologies. Furthermore, an in vivo test in New Zealand rabbits demonstrated that the HA scaffold with the flower-like surface resulted in more angiogenesis compared with the control scaffold. This copper-assisted hydrothermal deposition process provides a simple and controllable route for engineering a micro/nano-structured surface on the HA scaffolds, which has benefits in terms of angiogenesis and bone regeneration.
Synthesis, characterization and biological action of nano-bioglass ceramic particles for bone formation
Advances in synthesis of calcium phosphate crystals with controlled size and shape
Calcium phosphate (CaP) materials have a wide range of applications, including biomaterials, adsorbents, chemical engineering materials, catalysts and catalyst supports and mechanical reinforcements. The size and shape of CaP crystals and aggregates play critical roles in their applications. The main inorganic building blocks of human bones and teeth are nanocrystalline CaPs; recently, much progress has been made in the application of CaP nanocrystals and their composites for clinical repair of damaged bone and tooth. For example, CaPs with special micro- and nanostructures can better imitate the biomimetic features of human bone and tooth, and this offers significantly enhanced biological performances. Therefore, the design of CaP nano-/microcrystals, and the shape and hierarchical structures of CaPs, have great potential to revolutionize the field of hard tissue engineering, starting from bone/tooth repair and augmentation to controlled drug delivery devices. Previously, a number of reviews have reported the synthesis and properties of CaP materials, especially for hydroxyapatite (HAp). However, most of them mainly focused on the characterizations and physicochemical and biological properties of HAp particles. There are few reviews about the control of particle size and size distribution of CaPs, and in particular the control of nano-/microstructures on bulk CaP ceramic surfaces, which is a big challenge technically and may have great potential in tissue engineering applications. This review summarizes the current state of the art for the synthesis of CaP crystals with controlled sizes from the nano- to the macroscale, and the diverse shapes including the zero-dimensional shapes of particles and spheres, the one-dimensional shapes of rods, fibers, wires and whiskers, the two-dimensional shapes of sheets, disks, plates, belts, ribbons and flakes and the three-dimensional (3-D) shapes of porous, hollow, and biomimetic structures similar to biological bone and tooth. In addition, this review will also summarize studies on the controlled formation of nano-/microstructures on the surface of bulk ceramics, and the preparation of macroscopical bone grafts with 3-D architecture nano-/microstructured surfaces. Moreover, the possible directions of future research and development in this field, such as the detailed mechanisms behind the size and shape control in various strategies, the importance of theoretical simulation, self-assembly, biomineralization and sacrificial precursor strategies in the fabrication of biomimetic bone-like and enamel-like CaP materials are proposed. Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Three-dimensional printing of scaffolds with synergistic effects of micro-nano surfaces and hollow channels for bone regeneration
Bioinspired trimodal macro/ micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect
The effects of morphology on physicochemical properties, bioactivity and biocompatibility of micro-/nano-bioactive glasses
Study on the effect of PDA-PLGA scaffold loaded with islet cells for skeletal muscle transplantation in the treatment of diabetes
At present, islet cells transplantation was limited by the way in which islet cells are implanted into the body, their ability to adapt to the microenvironment and the maintenance time for relieving diabetic symptoms. In order to solve this problem, we made PDA-PLGA scaffold loaded with islet cells and used it for skeletal muscle transplantation to investigate its therapeutic effect in the treatment of diabetes. The PLGA scaffold was prepared by the electrospinning method, and modified by polydopamine coating. A rat diabetic model was established to evaluate the efficacy of PDA-PLGA scaffold loaded with RINm5f islet cells through skeletal muscle transplantation. The results showed that the PDA-PLGA scaffold has good biosafety performance. At the same time, transplantation of the stent to the skeletal muscle site had little effect on the serum biochemical indicators of rats, which was conducive to angiogenesis. The PDA-PLGA scaffold had no effect on the secretory function of pancreatic islet cells. The PDA-PLGA scaffold carrying RINm5f cells was transplanted into the skeletal muscle of type I diabetic rats. 1 week after the transplantation of the PDA-PLGA cell scaffold complex, the blood glucose of the treatment group was significantly lower than that of the model group (p &lt; 0.001) and lasted for approximately 3 weeks, which further indicated the skeletal muscle transplantation site was a new choice for islet cell transplantation in the future.
Microgrooves and microrugosities in titanium implant surfaces: an in vitro and in vivo evaluation
The physical characteristics of an implant surface can determine and/or facilitate osseointegration processes. In this sense, a new implant surface with microgrooves associated with plus double acid treatment to generate roughness was evaluated and compared in vitro and in vivo with a non-treated (smooth) and double acid surface treatment. Thirty disks and thirty-six conical implants manufactured from commercially pure titanium (grade IV) were prepared for this study. Three groups were determined, as described below: Group 1 (G1), where the samples were only machined; group 2 (G2), where the samples were machined and had their surface treated to generate roughness; and test group 3 (G3), where the samples were machined with microgrooves and the surface was treated to generate the roughness. For the in vitro analysis, the samples were submitted to scanning microscopy (SEM), surface profilometry, the atomic force microscope (MFA) and the surface energy test. For the in vivo analyses, thirty-six implants were placed in the tibia of 9 New Zealand rabbits in a randomized manner, after histological and histomorphometric analysis, to determine the level of contact between the bone and implant (BIC%) and the bone area fraction occupancy (BAFO%) inside of the threads. The data collected were statistically analyzed between groups (p < 0.05). The in vitro evaluations showed different roughness patterns between the groups, and the G3 group had the highest values. In vivo evaluations of the BIC% showed 50.45 ± 9.57% for the G1 group, 55.32 ± 10.31% for the G2 group and 68.65 ± 9.98% for the G3 group, with significant statistical difference between the groups (p < 0.0001). In the BAFO% values, the G1 group presented 54.97 ± 9.56%, the G2 group 59.09 ± 10.13% and the G3 group 70.12 ± 11.07%, with statistical difference between the groups (p < 0.001). The results obtained in the evaluations show that the surface with microgrooves stimulates the process of osseointegration, accelerating the healing process, increasing the contact between the bone and the implant and the area of new bone formation.
Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells
Type I collagen decorated nanoporous network on titanium implant surface promotes osseointegration through mediating immunomodulation, angiogenesis, and osteogenesis
3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin- polydopamine for enhanced osteogenic and anti-inflammatory activities
Modifications of parylene by microstructures and selenium nanoparticles: evaluation of bacterial and mesenchymal stem cell viability
Parylene-based implants or coatings introduce surfaces suffering from bacteria colonization. Here, we synthesized polyvinylpyrrolidone-stabilized selenium nanoparticles (SeNPs) as the antibacterial agent, and various approaches are studied for their reproducible adsorption, and thus the modification of parylene-C–coated glass substrate. The nanoparticle deposition process is optimized in the nanoparticle concentration to obtain evenly distributed NPs on the flat parylene-C surface. Moreover, the array of parylene-C micropillars is fabricated by the plasma etching of parylene-C on a silicon wafer, and the surface is modified with SeNPs. All designed surfaces are tested against two bacterial pathogens, Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). The results show no antibacterial effect toward S. aureus, while some bacteriostatic effect is observed for E. coli on the flat and microstructured parylene. However, SeNPs did not enhance the antibacterial effect against both bacteria. Additionally, all designed surfaces show cytotoxic effects toward mesenchymal stem cells at high SeNP deposition. These results provide valuable information about the potential antibacterial treatment of widely used parylene-C in biomedicine.
3D-printed scaffolds of mesoporous bioglass/gliadin/polycaprolactone ternary composite for enhancement of compressive strength, degradability, cell responses and new bone tissue ingrowth
Due to the increasing number of patients with bone defects, bone nonunion and osteo-myelitis, tumor and congenital diseases, bone repair has become an urgent problem to be solved.In this study, the 3D-printed scaffolds of ternary composites containing mesoporous bioglass fibers of magnesium calcium silicate (mMCS), gliadin (GA) and polycaprolactone (PCL) were fabricated using a 3D Bioprinter.The compressive strength and in vitro degradability of the mMCS/GA/PCL composites (MGPC) scaffolds were improved with the increase of mMCS content. In addition, the attachment and proliferation of MC3T3-E1 cells on the scaffolds were significantly promoted with the increase of mMCS content. Moreover, the cells with normal phenotype attached and spread well on the scaffolds surfaces, indicating good cytocompatibility. The scaffolds were implanted into the femur defects of rabbits, and the results demonstrated that the scaffold containing mMCS stimulated new bone formation and ingrowth into the scaffolds through scaffolds degradation in vivo. Moreover, the expression of type I collagen into scaffolds was enhanced with the increase of mMCS content.The 3D-printed MGPC scaffold with controllable architecture, good biocompatibility, high compressive strength, proper degradability and excellent in vivo osteogenesis has great potential for bone regeneration.
Controllable two-scale network architecture and enhanced mechanical properties of (Ti5Si3+TiBw)/Ti6Al4V composites
Novel Ti6Al4V alloy matrix composites with a controllable two-scale network architecture were successfully fabricated by reaction hot pressing (RHP). TiB whiskers (TiBw) were in-situ synthesized around the Ti6Al4V matrix particles, and formed the first-scale network structure (FSNS). Ti5Si3 needles (Ti5Si3) precipitated in the beta phase around the equiaxed a phase, and formed the secondary-scale network structure (SSNS). This resulted in increased deformation compatibility accompanied with enhanced mechanical properties. Apart from the reinforcement distribution and the volume fraction, the ratio between Ti5Si3 and TiBw fraction were controlled. The prepared (Ti5Si3 + TiBw)/Ti6Al4V composites showed higher tensile strength and ductility than the composites with a one-scale microstructure, and superior wear resistance over the Ti6Al4V alloy under dry sliding wear conditions at room temperature.
Mineralization of calcium phosphate controlled by biomimetic self-assembled peptide monolayers via surface electrostatic potentials
Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation