2H相MoS2具有独特的带隙结构和层间较弱的范德瓦尔斯力, 被广泛应用于润滑和光电催化领域。Wang等[5]通过水热一锅法将g-C3N4薄膜涂敷在MoS2纳米球的表面并成功构建了MoS2/g-C3N4 II型异质结, 应用于可见光下降解甲苯, 制备的异质结光催化剂的光催化活性比g-C3N4提升了1.3倍, 实验表明构建II型异质结可有效抑制g-C3N4表面光生载流子的复合, 其内部光生电子迁移至还原电位更低的MoS2表面并作为MoS2的电子补充剂参与光催化反应。II型异质结作为一种半导体改性方法, 可有效减少催化剂中光生载流子的复合, 但往往以牺牲氧化还原性更强的h+和光生电子为代价, 并不能促进光催化反应的高效进行[6-7]。Chu等[8-9]采用超声+溶剂热法制备了MoS2/g-C3N4的 2D/2D异质结, 光催化N还原反应中NH3的产率达到18.5 μg·h−1·mg-1, 比纯g-C3N4和MoS2表现出更优异的反应活性, 研究发现, MoS2/g-C3N4异质结交界处的Mo位点(0.53 eV)比MoS2边缘处的Mo位点 (0.87 eV)的N氢化稳定自由能(
本工作以廉价的(NH4)6Mo7O24·4H2O和CS(NH2)2作为Mo源和S源, 采用水热法制备花簇状MoS2微球, 并采用超声沉积+煅烧法构建了MoS2/g-C3N4 S型异质结, 通过引入MoS2, 不仅可以调整禁带宽度, 扩大可见光的吸收范围, 同时可以利用异质结由能带交错而产生的电荷传输间接减少光生载流子的复合, 进而促进光催化反应, 并从内建电场形成的角度分析了异质结电荷的传输及光催化降解机理。
1 实验方法
1.1 光催化剂制备
MoS2和g-C3N4光催化剂的制备: 称取40 g尿素放入200 mL带盖刚玉坩埚中, 并用锡箔纸封住, 置于马弗炉中。以5 ℃/min的升温速率逐渐升温至550 ℃并保温240 min, 取出、研磨得到淡黄色粉末状g-C3N4。将2.471 g钼酸铵和5.32 g硫脲溶于80 mL去离子水中, 超声处理3 h后置于磁力搅拌器上再搅拌3 h后装入100 mL反应釜中, 在200 ℃反应24 h, 分离上层清液, 得到的黑色粉末用去离子水/乙醇反复洗涤5次, 将pH调至7制得黑色粉末状MoS2, 将制得的g-C3N4 和MoS2分别在氮气氛围下以5 ℃/min的速率升温至300 ℃并保温3 h, 并将煅烧后的g-C3N4记为GCD、煅烧后的MoS2记为MOS。
MoS2/g-C3N4复合光催化剂的制备: 按照MoS2质量分数为1%、3%、5%、7%称取MoS2和g-C3N4粉末, 将g-C3N4粉末置于100 mL烧杯中, 分别加入30 mL乙醇和40 mL去离子水, 超声3 h后再搅拌3 h, 经过滤置于80 ℃干燥箱烘干8 h, 得到MoS2/g-C3N4复合光催化剂。将复合光催化剂在氮气氛围下以5 ℃/min的速率升温至300 ℃并保温3 h, 制得MoS2/g-C3N4异质结结构光催化剂, 并标记为x%MGCD(其中, x%分别为1%、3%、5%和7%)。
1.2 光催化性能及自由基捕获
采用20 mg/L的RhB溶液为模拟降解物进行光催化实验、稳定性及自由基捕获实验, 催化剂投加量为20 mg/L。无光照条件下搅拌30 min达到吸脱附平衡后分别取4 mL左右反应液, 开启光源, 每隔10 min取样, 经离心后测量上层清液的吸光度。收集暗反应阶段吸出的悬浊液和光反应完成后反应瓶中剩余悬浊液, 经抽滤、洗涤后重新进行第二次光催化实验, 循环测试5次来评价样品的光催化稳定性, 并通过添加相应的猝灭剂BQ (O2-)、KI(h+)和TBA(·OH)参与载流子捕获实验来探究光催化机理。
1.3 表征测试
采用德国Bruker D8-ADVANCE型衍射仪进行XRD表征; 采用德国蔡司Sigma300型场发射扫描电镜和日本日立 HT7800 透射电镜观察表面微观形貌; 分别采用日本日立U-3900型紫外-可见漫反射光谱仪和U-4600荧光光谱仪测试DRS和PL荧光光谱。采用FT-IR1200型傅里叶变换红外光谱仪测试FT-IR光谱。采用北京泊菲莱PCX-50C型多通道光化学反应器进行光催化反应, 光照强度260 mW/cm2; 采用荷兰IVIUM电化学工作站测试样品的光电性能。
2 结果与讨论
2.1 物相、微观形貌
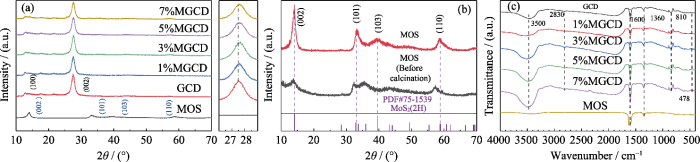
不同样品的XRD谱图由图1(a)所示, 其中2θ=13.1°和27.3°左右的衍射峰来自g-C3N4的(100)和(002)晶面衍射, 分别对应着三嗪环面内的平铺和氮化碳的层间堆叠[11]。随着MoS2含量增大, 2θ=27.3°处衍射峰逐渐发生右移, 说明随着MoS2的引入, g-C3N4分子的晶面间距有所缩小, 且该衍射峰的半峰宽逐渐变大, g-C3N4晶粒度逐渐变小, 这有助于增大光催化剂的比表面积并暴露更多的反应活性位点, 促进光催化反应的高效进行。煅烧前后MoS2样品的XRD图谱如图1(b)所示, 其中2θ=14.1°、33.7°、39.5°和58.8°处衍射峰分别对应MoS2的(002)、(101)、(103)、(110)晶面, 与标准卡片(PDF#75-1539)相符合[10], 可以看出经保护气氛下煅烧, MoS2的结晶度明显提高并具有(002)晶面择优取向, 这有助于提高MoS2的致密度和稳定性并巩固异质结结构, 煅烧后MoS2没有出现杂峰, 也体现出MoS2良好的热稳定性。
图1
图1
不同样品的XRD谱图(a), 煅烧前后的MoS2的XRD谱图(b)和样品的红外光谱图(c)
Fig. 1
XRD patterns of different samples (a), XRD patterns of MoS2 samples before and after calcination (b), and FT-IR spectra of samples (c)
不同样品的红外光谱图如图1(c)所示, 指纹区478 cm-1处的吸收峰归属于MoS2中Mo-S键垂直于基面振动[12], 810 cm-1处的吸收峰归属于g-C3N4中庚三氮环的面外弯曲振动, 1360和1600 cm-1处出现的吸收峰归属于碳氮杂环的弯曲振动[13]。官能团区中2830 cm-1处的宽峰对应缔合态N-H键的伸缩振动模式, 3500 cm-1处的吸收峰对应缔合态O-H键的伸缩振动模式, 来自于超声时样品表面的吸附氧。通过对比可以看出, 随着MoS2含量的增加, 810 cm-1处的振动吸收峰强度增大, 表明MoS2的复合明显影响了g-C3N4分子所处的化学环境, 也充分说明二者成功构建了异质结, 这与XRD分析结果一致。
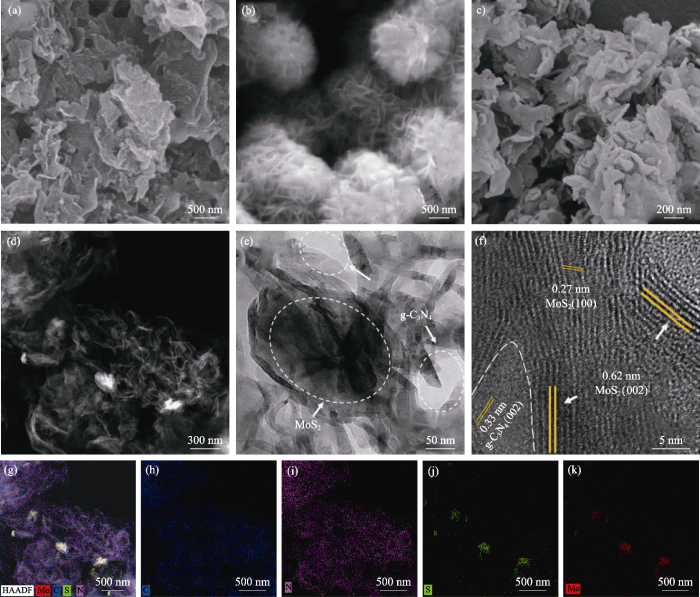
不同样品的微观形貌如图2所示。由图2(a)所示, GCD呈现出不规则的花片状, 这是由g-C3N4纳米片通过物理吸附的方式团聚成块并降低自身表面能达到的稳定状态。由图2(b)可见, 合成的MoS2外观呈花球状, 花球内部是为了降低表面能而自组装卷曲团聚而成的褶皱状纳米片[14]。图2(c, d)给出了5%MGCD的表面形貌照片, g-C3N4呈纳米片状, 未观察到MoS2, 这可能是MoS2含量少、分散所致。5%MGCD样品的HR-TEM照片如图2(e, f)所示, 从图2(e)中可以观察到g-C3N4呈纳米薄片状, MoS2微球破碎后的纳米片分布在g-C3N4表面。从图2(f)观察到MoS2的晶格条纹, 其晶格间距对应(002)晶面, 同时在其周边观测到致密的g-C3N4晶格条纹, 晶格间距为0.33 nm, 对应(002)晶面, 两者紧密结合, 表明二者成功构建了异质结结构。如元素分布(图2(g~k))所示, 复合样品中MoS2花球经超声-煅烧后发生破碎, 形成的纳米片层分散、点缀修饰在g-C3N4表面, 并无杂相, 也充分显示了MoS2的分散性和热稳定性良好。
图2
图2
g-C3N4 (a), MoS2 (b)和5%MGCD(c, d)的SEM照片、HR-TEM照片(e, f)、元素分布总图(g)及其元素C(h), N(i), S(j), Mo(k)分布图
Fig. 2
FE-SEM images of g-C3N4 (a), MoS2 (b) and FE-SEM images (c, d), HR-TEM images (e, f),element mapping (g) and C (h), N (i), S (j), Mo (k) mapping of 5%MGCD
2.2 光电学性能
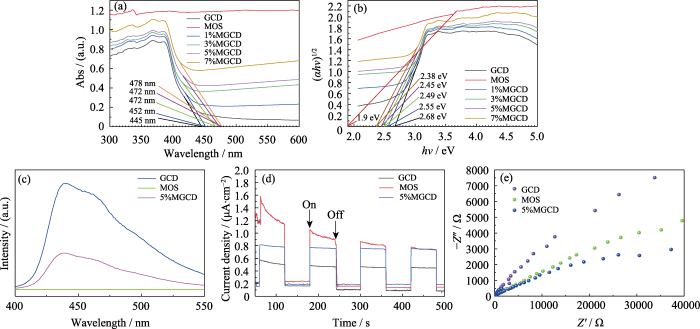
图3(a)是样品的紫外-可见吸收光谱图, 随着MoS2引入量增大, 样品的吸收带边逐渐发红移。图3(b)是结合Tauc-plot公式得到的样品的带隙图, 当MoS2引入量为5%和7%时, 样品带隙分别为2.45和2.38 eV, 说明MoS2与g-C3N4复合后, 样品的带隙更窄, 光生电子的跃迁也更加容易。借助Tauc法并结合能带公式
图3
图3
样品的紫外-可见吸收光谱图(a), Tauc曲线(b), 荧光光谱图(c)、瞬态光电流响应图谱(d)及EIS图谱(e)
Fig. 3
UV-Vis (a) spectra, Tauc curves (b), PL spectra (c), instantaneous meter photocurrents (d), and EIS (e) patterns of samples
Colorful figures are available on website
为了进一步研究MoS2与g-C3N4接触界面的电荷转移及光生载流子的分离状况, 测试了样品的光电性能。图3(c)为不同样品在370 nm激发波长下的PL谱图, GCD和5%MGCD样品的发射峰都集中在450 nm附近, 5% MGCD的荧光强度远小于g-C3N4, 说明其载流子的复合程度显著下降, 进一步说明MoS2与g-C3N4成功耦合, 并且由其交错的能带结构和能级差异引导光生电子在不同的HOMO和LUMO能级中传导, 减少光生载流子的复合。样品的瞬态光电流响应如图3(d)显示, 相较于纯g-C3N4, 异质结催化剂的光电流强度有明显提高, 这也意味着复合材料中载流子的分离率更高, 寿命更长, 更有助于光催化反应的高效进行[17-18]。电化学交流阻抗(EIS)图谱常被用于研究半导体光催化材料内部载流子的分离与载流子传质的特性, 其中Nyquist圆弧半径大小代表半导体电极表面电荷的转移的阻力[19], 5%MGCD异质结样品的Nyquist圆弧半径比GCD样品的更小(图3(e)), 说明5%MGCD异质结催化剂电极/电解质液表面的电荷的传输阻抗更小, 更有助于载流子有效转移[20-21]。
2.3 光催化性能及光催化稳定性研究
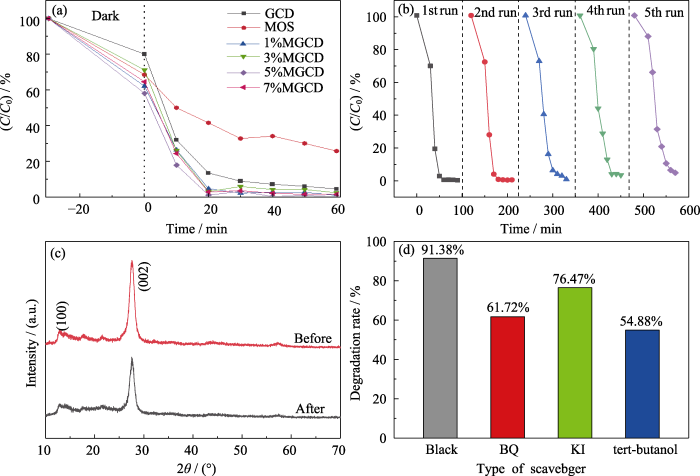
图4(a)为不同样品对RhB降解率对比图, 可以看到复合光催化剂的光催化效率均优于纯相g-C3N4和MOS。且当MoS2引入量为5%时, 催化20 min样品对RhB的降解率可以达到99%, 较纯相g-C3N4明显提升。良好的稳定性是评价光催化剂综合性能的重要标准[22-23], 5%MGCD样品的降解率随时间变化图(图4(b)), 循环使用5次时, 样品对RhB的降解率仍能保持在95.2%, 说明样品具有良好的光催化稳定性。由样品使用前后的XRD图谱(图4(c))可知, 循环实验五次以后光催化剂g-C3N4的(100)和(002)晶面的衍射峰仍保持较明显的峰型, 说明该复合光催化剂具有良好的相结构稳定性[24-25]。
图4
图4
(a) 不同光催化剂样品对RhB随时间变化的降解率对比图, (b) 5%MGCD的循环降解率随时间变化图, (c) 5%MGCD循环降解实验前后的XRD谱图, 以及(d) 自由基捕获实验图
Fig. 4
Degradation rate of different photocatalyst samples (a), cyclic degradation rate of 5%MGCD with time (b), XRD patterns of 5%MGCD before and after circulation experiment (c), and histogram of free radical trapping experiment (d)
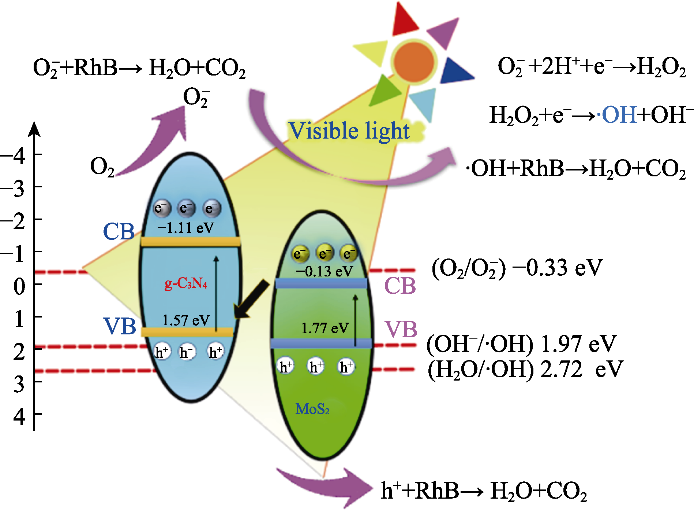
为了探究光催化剂的光催化机理, 对光催化降解过程进行了自由基捕获实验, 结果如图4(d)所示, 降解过程中添加BQ、TBA和KI活性物种牺牲剂后, 5%MGCD异质结催化剂对RhB的光催化降解率分别下降到61.72%、54.88%和76.47%, 在降解过程中, BQ和TBA对相应活性物种的猝灭效应较为明显, 说明起降解作用的主要活性物种为·OH和O2-, h+对光催化降解的贡献较小。
2.4 光催化机理研究
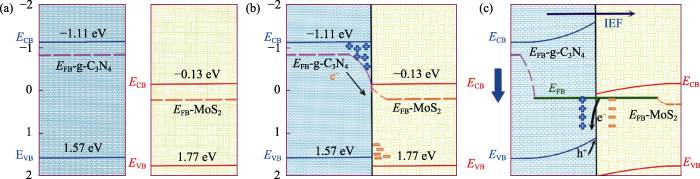
结合自由基捕获实验, 以BQ为牺牲剂可以有效抑制O2-生成, 说明g-C3N4与MoS2形成的异质结能够有效保留g-C3N4中还原性更强的光生电子并促进O2发生还原反应,生成活性物种O2-, 其载流子传输途径不同于传统的Z型/II型异质结。如图5所示, 通常半导体的平带电位比N型半导体导带底部低0.1~0.3 eV[26]。图5(a)给出了g-C3N4和MoS2形成界面的费米能级及能带变换情况, 当受到一系列大于两者带隙的可见光照射后, 光生电子由稳态变成激发态并发生跃迁, 受费米能级差异的影响, 电荷会从g-C3N4流入MoS2(如图5(b)所示), g-C3N4区的费米能级逐渐降低, 同时MoS2区的费米能级逐渐升高, 并在交界处生成空间电荷区, 伴随产生的内建电场方向由g-C3N4区指向MoS2区(如图5(c)所示), 沿g-C3N4区的电荷电势至MoS2不断下降, 同时电子的电势能-qV沿相同的方向不断增大, 内建电场会在费米能级弯曲的同时拉高g-C3N4的能带, 拉低MoS2能带, 因此, g-C3N4区的光生电子需要克服电势能垒才能回落至MoS2区, 即抑制电荷的输出, 同时允许空穴输入。MoS2区的电荷为高势能态, 能带的下弯允许电子的输出, 同时抑制空穴输入, 氧化电位较低的g-C3N4-VB区h+和MoS2-CB区的光生电子会优先复合并保留具有更强氧化还原电位的h+和e-[27-28]。
图5
图5
(a) 异质结能带图, (b) 样品异质结的构建及能费米能级的弯曲, (c) 内建电场引起的能带弯曲图
Fig. 5
Energy bands diagram of heterojunction (a), construction of heterojunction of sample and bending of Fermi energy level (b), and diagram of energy band bending caused by built-in electric field (c)
结合上述实验结果分析进一步提出光催化降解机理, 如图6所示, g-C3N4与2H相MoS2紧密结合形成S型异质结, 光照使半导体的光生电荷发生跃迁, 内建电场会使MoS2导带处的电子优先回落至g-C3N4的价带, 保留了MoS2价带处氧化性更强的光生空穴[29]。在g-C3N4导带处的电荷具有较强的还原性, 能够将吸附于催化剂表面的氧气还原成活性物种O2-并降解罗丹明B, 同时, O2-与溶液中的H+、e-结合生成双氧水也能促进·OH的生成, 并共同参与反应[30-31]。在可见光照射下, MoS2的电子在电场作用下迁移至g-C3N4并与光生空穴复合, 同时, 大量g-C3N4空穴在电场牵引下向MoS2更强氧化电位移动并更有效促进 RhB降解反应。两种常规N型半导体的载流子为光生e-。在载流子捕获实验中, h+的猝灭效应不显著, 可能是h+的数量少导致的。
图6
3 结论
通过简便易操作的超声沉积+煅烧法将g-C3N4、MoS2两种半导体耦合并巩固, 制备了g-C3N4/MoS2 S型异质结。S型异质结的构筑有效提升了样品的光催化活性, 当MoS2含量为5%时, 光照20 min复合光催化剂对RhB的降解率达到99%, 比纯相氮化碳有明显提升。不同于II型和Z型异质结, 内建电场的加持下, S型异质结的电子-空穴传输途径有效抑制了g-C3N4光生载流子的复合, 并保留了强氧化性的h+和强还原性e-用于反应生成O2-、·OH等活性物种协助降解RhB。通过充分利用MoS2良好的分散性及热稳定性的特点, 对g-C3N4实现了少量低成本的复合改性工作。
参考文献
Self-assembled hierarchical carbon/g-C3N4 composite with high photocatalytic activity
Synthesis of novel MoS2/g-C3N4nanocomposites for enhanced photocatalytic activity
Sulfate modified g-C3N4 with enhanced photocatalytic activity towards hydrogen evolution: the role of sulfate in photocatalysis
New insight into the mechanism of enhanced photo-Fenton reaction efficiency for Fe-doped semiconductors: a case study of Fe/g-C3N4
Fabrication of MoS2@g-C3N4 core-shell nanospheresfor visible light photocatalytic degradation of toluene
A combination of two visible-light responsive photocatalysts for achieving the Z-Scheme in the solid state
The light reaction in natural photosynthesis is generally recognized as one of the most efficient mechanisms for converting solar energy into other energy sources. We report herein on a novel strategy for generating H(2) fuel via an artificial Z-scheme mechanism by mimicking the natural photosynthesis that occurs in green plants. Designing a desirable photocatalyst by mimicking the Z-scheme mechanism leads to a conduction band that is sufficiently high to reduce protons, thus decreasing the probability of charge recombination. We combined two visible light sensitive photocatalysts, CdS and carbon-doped TiO(2), with different band structures. The used of this combination, that is, CdS/Au/TiO(1.96)C(0.04), resulted in the successful transfer of photogenerated electrons to a higher energy level in the form of the letter 'Z'. The system produced about a 4 times higher amount of H(2) under irradiation by visible light than CdS/Au/TiO(2). The findings reported herein describe an innovative route to harvesting energy by mimicking natural photosynthesis, and is independent of fossil fuels.
Reduced graphene oxide as a solid-state electron mediator in z-scheme photocatalytic water splitting under visible light
The effectiveness of reduced graphene oxide as a solid electron mediator for water splitting in the Z-scheme photocatalysis system is demonstrated. We show that a tailor-made, photoreduced graphene oxide can shuttle photogenerated electrons from an O(2)-evolving photocatalyst (BiVO(4)) to a H(2)-evolving photocatalyst (Ru/SrTiO(3):Rh), tripling the consumption of electron-hole pairs in the water splitting reaction under visible-light irradiation.
Two-dimensional (2D)/2D interface engineering of a MoS2/C3N4 heterostructure for promoted electrocatalytic nitrogen fixation
Constructing highly oriented configuration by few-layer MoS2: toward high-performance lithium-ion batteries and hydrogen evolution reactions
Constructing three-dimensional (3D) architecture with oriented configurations by two-dimensional nanobuilding blocks is highly challenging but desirable for practical applications. The well-oriented open structure can facilitate storage and efficient transport of ion, electron, and mass for high-performance energy technologies. Using MoS2 as an example, we present a facile and effective hydrothermal method to synthesize 3D radially oriented MoS2 nanospheres. The nanosheets in the MoS2 nanospheres are found to have less than five layers with an expanded (002) plane, which facilitates storage and efficient transport of ion, electron, and mass. When evaluated as anode materials for rechargeable Li-ion batteries, the MoS2 nanospheres show an outstanding performance; namely, a specific capacity as large as 1009.2 mA h g(-1) is delivered at 500 mA g(-1) even after 500 deep charge/discharge cycles. Apart from promising the lithium-ion battery anode, this 3D radially oriented MoS2 nanospheres also show high activity and stability for the hydrogen evolution reaction.
High-throughput production of ZnO-MoS2 graphene heterostructures for highly efficient photocatalytic hydrogen evolution
The nucleation and grain growth that occur during solidification have been extensively examined, but insight into the influence of an external field on the formation of heterogeneous crystal nuclei above the liquidus remains unclear in the peritectic refinement mechanism. In this work, we studied the effect of cooling rate above the liquidus on the formation of primary Al3Zr and grain refinement in Al-0.2%Zr alloys with inter-cooling annular electromagnetic stirring (IC-AEMS). The results show that the size and distribution of primary Al3Zr are greatly improved, and the morphology transformed from large plate/blocky shapes without IC-AEMS to small blocks with IC-AEMS. Meanwhile, above the liquidus, the addition of an Al-Zr master alloy to pure Al alone did little to enhance the refinement, but after IC-AEMS, the grains were refined dramatically. The refinement result seems to be explained by two hypotheses of pre-nucleation and explosive nucleation.
Photocatalytic activity enhancement of g-C3N4/BiOBr in selective transformation of primary amines to imines and its reaction mechanism
Anilinonaphthalene sulfonate binds to central cavity of humanhemo globin
Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation
Enhancement of visible light photocatalytic activities via porous structure of g-C3N4
Tungstenic acid induced assembly of hierarchical flower-like MoS2 spheres
A Z-scheme mechanism of N-ZnO/g-C3N4 for enhanced H2 evolution and photocatalytic degradation
Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two-dimensional nanojunctions for enhanced solar hydrogen generation
Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction
Layered nanojunctions for hydrogen-evolution catalysis
BiVO4 quantum tubes loaded on reduced graphene oxide aerogel as efficient photocatalyst for gaseous formaldehyde degradation
Capacitive deionization with MoS2/g-C3N4 electrodes
Preparation of MoS2 nanofibers by electrospinning
Formation of MoS2 inorganic fullerenes (IFs) by the reaction of MoO3 nanobelts and S.
Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 nanospheres modified porous g-C3N4
Noble metal-free Ni(OH)2-g-C3N4 composite photocatalyst with enhanced visible- light photocatalytic H2-production activity
S-scheme Ti0.7Sn0.3O2/g-C3N4 heterojunction composite for enhanced photocatalytic pollutants degradation
Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution
Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production
A step-by-step synergistic stripping approach toward ultra-thin porous g-C3N4 nanosheets with high conduction band position for photocatalystic CO2 reduction
In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis