为此, 研究者开始尝试利用带有机基团的硅氧烷来制备有机硅气凝胶, 以改善纯SiO2气凝胶力学性能差等问题。将甲基、苯基、乙烯基等有机基团引入到Si-O-Si的无机骨架中, 既增大分子链间的空间, 赋予气凝胶一定柔性, 又可取代分子中部分未反应完全的亲水羟基, 从而有效抑制气凝胶干燥过程中的收缩问题, 形成的有机-无机杂化网络兼具有机气凝胶和无机气凝胶的特性[13]。其中最具代表性的如Kanamori等[14]和Rao等[15]以三官能度的甲基三甲氧基硅烷为前驱体, 通过使用表面活性剂及酸碱两步催化法制得了兼具高透光率、低热导率以及优良力学特性的有机硅气凝胶。Zu等[16]采用甲基乙烯基二甲氧基硅烷为前驱体, 在DTBP引发剂的作用下先对乙烯基进行聚合, 然后再通过溶胶-凝胶方式制备了带甲基的双交联网络结构气凝胶, 双交联结构使气凝胶具备优良的力学性能和隔热性能。引入有机基团方式可有效改善SiO2气凝胶干燥成本高和力学性能差等问题, 但同时也会降低气凝胶的耐温性能, 尤其是在高温隔热应用领域, 其有机组分热解过程及高温无机化转变过程值得深入研究。
本研究基于有机-无机杂化提升气凝胶力学性能的方法, 采用含甲基、乙烯基侧链的VTMS和VMDMS双前驱体制备有机硅气凝胶, 探究其微观孔结构的调控方法及其对气凝胶压缩回弹性能的影响。此外, 详细分析了气凝胶在有氧和无氧环境下的高温无机化转变过程, 并对无氧环境下形成的无定形Si-O-C和SiC进行了耐高温氧化性能研究, 以期为有机硅气凝胶的高温应用和耐高温氧化无机气凝胶的制备提供参考。
1 实验方法
1.1 实验原料
乙烯基三甲氧基硅烷(VTMS)和乙烯基甲基二甲氧基硅烷(VMDMS), 购自上海贤鼎生物科技有限公司; 冰乙酸、尿素、十六烷基三甲基氯化铵(CTAC), 购自上海凌峰化学试剂有限公司。上述原料均为分析纯。
1.2 气凝胶的制备
将VTMS、VMDMS、CTAC、尿素和稀乙酸加入到烧杯中, 并在室温下搅拌0.5~1 h得到有机硅溶胶, 其中VTMS与VMDMS的摩尔比为1、2、3、4。将上诉溶胶置于80 ℃的真空干燥烘箱中凝胶老化24 h得到湿凝胶, 然后分别用去离子水和乙醇充分洗涤置换湿凝胶3~5次, 最后在室温下干燥12~36 h得到气凝胶。根据前驱体中VTMS/VMDMS的摩尔比, 分别标记为V/VM-1、V/VM-2、V/VM-3、V/VM-4。
1.3 分析与检测
采用扫描电子显微镜(NOVA Nano 450)观察样品的微观形貌; 利用热导仪(Netzsch HFM 446)测量样品的热导率; 利用能谱仪(Avance-300, Bruker)测试样品的固态29Si 核磁共振谱; 采用电子万能试验机(Instron 3367)测试样品的压缩回弹性能; 采用TG-IR分析仪分析有机硅气凝胶的裂解产物(SDT Q600 & Spectrum 100), 测试温度30~850 ℃, 升温速率为10 ℃/min; 用X射线衍射仪(D/max2550VB/PC)测试样品的晶相。
2 结果与讨论
2.1 气凝胶的制备及其微观结构调控
VTMS/VMDMS两种前驱体在酸性催化剂(乙酸)作用下进行充分的水解脱醇反应, 形成含有大量Si-OH的活性中间体, 紧接着在尿素高温分解产生的碱性环境下, 活性中间体的Si-OH发生脱水缩合反应, 形成具有三维多孔网状的骨架结构; 不同摩尔比前驱体制备的有机硅气凝胶密度在0.12~ 0.16 g/cm3范围, 热导率在0.028~0.032 W/(m·K)。图1(b)为不同摩尔比VTMS/VMDMS制备气凝胶的红外谱图, 1135和1039 cm-1两处的特征峰, 归属于有机硅的主链结构Si-O-Si[17], 可以看出随着三官能度VTMS比例增大, 硅烷分子间的交联程度增加, 所呈现的Si-O-Si红外特征峰也更明显; 此外, 2957 cm-1处的-CH3特征峰和1267与817 cm-1处的Si-C特征峰逐渐减弱, 主要是由于VMDMS比例降低[18], 甲基含量减少导致; 在1604、1408 cm-1处的特征峰归属于乙烯基特有的C=C和=CH2特征峰[19], 随VTMS/ VMDMS摩尔比的变化, 乙烯基总量应保持不变, 但乙烯基特征峰却呈减弱趋势, 这可能与硅烷分子间交联程度增加有关。
图1
图1
溶胶-凝胶过程示意图(a), 气凝胶样品的红外谱图(b)、核磁谱图(c)和SEM照片(d~g)
Fig. 1
Schematic diagram of Sol-Gel process (a), IR spectra (b), NMR spectra (c) and SEM images (d-g) of aerogel samples
Molar ratios of VTMS/VMDMS for aerogels (d-g) are 1, 2, 3, 4, respectively
图1(d~g)为不同摩尔比VTMS/VMDMS制备气凝胶的SEM照片, 由图可知, 随着VTMS/VMDMS摩尔比增大, 气凝胶颗粒逐渐减小, 且颗粒间堆积更紧密, 但摩尔比增大到2以上, 这种变化趋势并不明显, 这与硅烷分子间的交联结构有关。结合前驱体溶胶-凝胶过程示意图(图1(a))可知, VTMS含有三个可水解官能团, 水解得到的高活性中间产物进行分子间的脱水缩合反应, 会形成初级凝胶粒子, 而初级凝胶粒子生长到一定大小就会发生链终止, 因此初级凝胶粒子较小; 而VMDMS仅含两个可水解官能团, 前驱体经过水解后, Si-O-Si主链可进行二维拓展, 因此形成的初级凝胶粒子较大。较小的初级凝胶粒子相互缠绕形成的三维网络结构比较紧密, 颗粒间孔隙比较小; 而较大初级粒子形成的三维网络结构比较疏松, 颗粒间孔隙较大。上述分析与图1(c)核磁分析结果相对应, 当VTMS/VMDMS摩尔比值增大到2以上, D峰所对应的(CH3)(C=C)Si(OSi)2二维拓展结构趋于稳定, 因此气凝胶微观形貌变化也趋于稳定。
2.2 气凝胶的压缩回弹性能
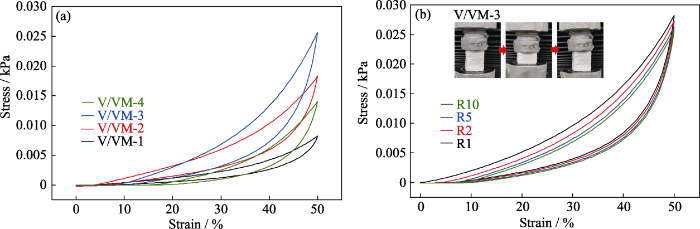
图2(a)所示为气凝胶样品(17 mm×17 mm× 21.5 mm)经过10次循环压缩的应力-应变曲线, 压缩应变量为50%。由图可知, 经10次循环压缩后气凝胶未出现坍塌或变形, 去除压力后均能很好地恢复原始尺寸。此外, 随VTMS/VMDMS摩尔比从1增大到4, 气凝胶的应力呈现先增加后减小的趋势, 其中样品V/VM-3应力最大, 为0.0256 kPa。应力的变化趋势可以归因于气凝胶的微观结构变化:随着三官能度前驱体相对摩尔比例的增加, 气凝胶颗粒呈现减小趋势, 颗粒间堆积紧密, 形成的三维网状骨架强度增大, 因此在外力压缩下应力增加。继续增加三官能度前驱体的比例, 气凝胶的颗粒堆积更加致密, 三维网状骨架开始失去柔韧性, 导致外力压缩下回弹力性能下降[22]。
图2
图2
气凝胶样品的循环压缩应力-应变曲线
Fig. 2
Cyclic compression stress-strain curves of aerogels
(a) Stress-strain curves after 10-cycle compression; (b) Cyclic compression stress-strain curves of sample V/VM-3 Colorful figures are available on website
图2(b)为样品V/VM-3的1、2、5和10次重复压缩试验的应力-应变曲线, 每次压缩应变设定为50%。可以看出, 随着压缩次数从1增加到10, 对应50%应变的应力仅从0.027 kPa降到0.025 kPa, 说明多次循环压缩并没有破坏气凝胶的空间结构, 气凝胶基本恢复原状, 并保持良好的弹性性能。
2.3 气凝胶的高温结构演变
2.3.1 空气氛围
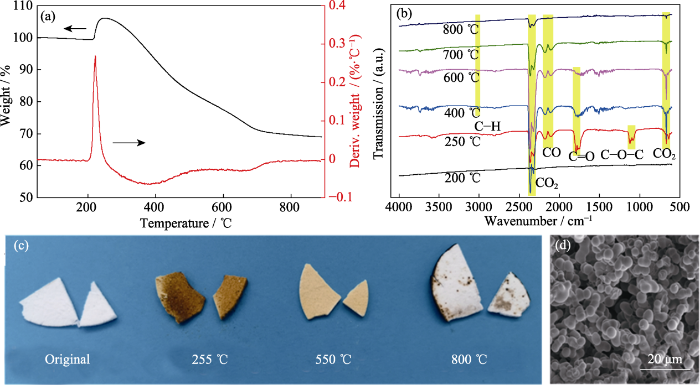
实验通过TG-IR联用形式探测气凝胶在30~800 ℃(空气气氛)范围内热解气的生成情况, 以此探究气凝胶在空气氛围下的高温结构演变过程。图3(a)为气凝胶样品V/VM-3在空气氛围下的TG-DTG曲线。由TG曲线可知, 样品在210~255 ℃范围内出现一个氧化还原峰, 质量增加约5%, 结合250 ℃裂解气红外曲线(图3(b)), 在1797和1121 cm-1处分别出现了C=O和C-O-C的吸收峰, 初步分析这一阶段主要发生了乙烯基的氧化还原反应[23], 生成了部分高活性碳氧中间产物, 并伴随少量CO和CO2裂解气; 随着温度升高, 大量中间产物被继续氧化成CO、CO2、H2O等小分子物质释放, 导致质量快速下降; 到400 ℃时, 裂解气红外曲线在3017 cm-1处出现C-H的特征峰, 且C=O和C-O-C的吸收峰基本消失, 说明此时有机硅侧链上的-CH3等有机基团被氧化分解成碳氢类小分子物质, 而乙烯基的氧化过程基本结束, 此时释放的大量CO、CO2、H2O等气体主要源于游离碳和裂解中间产物氧化, 此过程伴随着主链Si-O-Si断裂和重排, 质量继续减小。到700 ℃时, 甲基和中间产物氧化分解、游离碳氧化开始减少, 直到800 ℃有机部分的氧化基本完成, 气凝胶转化为无机SiO2气凝胶, 其残重保持在69%左右。
图3
图3
样品V/VM-3在空气氛围下的TG-IR谱图(a, b), 经不同温度处理后的实物图(c)和在800 ℃处理后的SEM照片(d)
Fig. 3
(a, b) TG-IR spectra of sample V/VM-3 tested in air; (c) Photographs of sample V/VM-3 after heat-treated at different temperatures; (d) SEM image of sample V/VM-3 after heat-treated at 800 ℃
2.3.2 氮气氛围
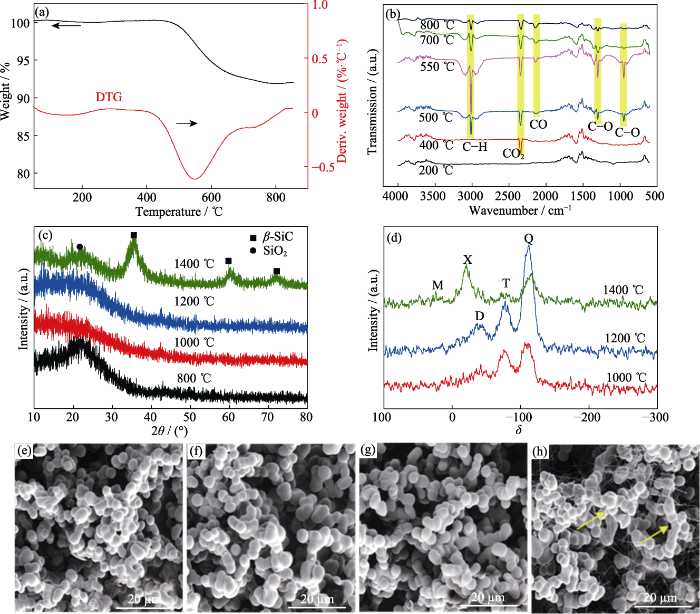
同样采用TG-IR联用形式探测气凝胶在30~ 800 ℃(氮气气氛)范围内裂解气的生成情况。图4(a)为样品V/VM-3的TG-DTG曲线, 图4(b)为样品V/VM-3在升温过程中裂解气的红外谱图。由图可知, 200 ℃时, 裂解气在3780 cm-1处出现了水羟基伸缩振动峰, 在3500~4000 cm-1和1300~1800 cm-1附近出现明显的毛刺状吸收峰, 这是样品中的水分对红外吸收峰的干扰所致[24]。当温度升至400 ℃, 在2342 cm-1处出现明显CO2特征峰, 这是由少量未完全水解的甲氧基高温裂解产生的[25], 但样品未出现明显失重。从480 ℃开始样品质量开始迅速下降, 结合图4(b)中500 ℃裂解气红外曲线可知, 有机侧链裂解反应和主链的断裂重排开始全面发生, 在3017 cm-1附近出现C-H吸收峰, 说明此时甲基等侧链已经裂解为甲烷等低分子烃类物质[26]; 此外, 在1296和948 cm-1处出现了C-O吸收峰, 说明此时主链中的Si-O-Si开始断裂, 和有机侧链反应生成一些碳氧类的中间产物[27]以及少量CO。升到 550 ℃时, 裂解反应达到顶峰, 此时碳氢和碳氧类的裂解产物含量达到最大, 这与图4(a)所示DTG曲线对应。随着温度进一步升高, 裂解反应速率开始下降, 升至700 ℃时基本没有碳氧类中间产物的挥发, 升到800 ℃时裂解过程进入收尾阶段, 尽管仍有少量CH4、CO2、CO和低分子烃类物质在缓慢释放, 但其残重已基本稳定在92%左右。
图4
图4
样品V/VM-3在N2氛围下的TG-IR谱图(a, b), 在不同温度处理后的XRD谱图(c)、核磁谱图(d)和SEM照片(e~h)
Fig. 4
(a, b) TG-IR spectra of sample V/VM-3 under N2 atmosphere; (c) XRD patterns, (d) NMR spectra and (e-h) SEM image of sample V/VM-3 after heat-treated at different temperatures
(e) 800 ℃; (f) 1000 ℃; (g) 1200 ℃; (h) 1400 ℃
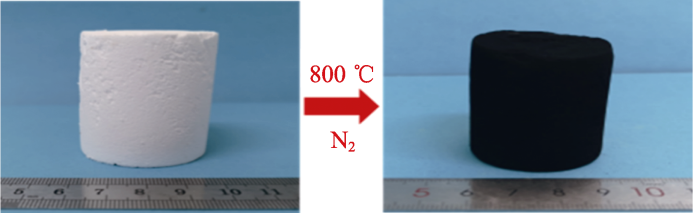
实验进一步探究了气凝胶在更高温度下的高温结构变化。将气凝胶样品V/VM-3分别置于800、1000、1200和1400 ℃的N2环境中碳化2 h, 图4(c, d)为碳化后样品的XRD和核磁谱图。由XRD谱图可知, 经800 ℃碳化后的样品在2θ=22°处出现明显SiO2特征峰, 说明800 ℃处理后的有机硅气凝胶已基本转化为无机SiO2, 结合处理前后实物图(图5)表明除无机SiO2以外, 还残余大量游离碳使样品呈黑色。而经1000和1200 ℃处理后样品的SiO2特征峰基本消失, 在核磁谱图的δ=-111, -77, -41处分别出现Si原子的Q峰、T峰和D峰, 分别对应SiO4、SiCO3和SiC2O2的硅氧碳无定形结构, 可以推测在1000~1200 ℃(N2)环境下, 无机结晶态的SiO2会首先转化为无定形态SiO4结构, 然后与游离碳发生碳热还原反应, 使碳原子取代SiO4中的1~2个氧原子而形成SiCO3和SiC2O2两种Si-O-C结构, 且温度越高这种反应趋势越明显。1400 ℃处理后, 样品在2θ=35.66°、60.29°、72.29°处出现明显的β-SiC晶体特征峰[28-29], 结合核磁谱图结果发现在δ=-20处出现明显的X峰, 对应SiC结构, 并在δ=-23处出现了与SiC3O结构对应的微弱M峰, 由此可以断定1400 ℃高温下, 碳热还原反应进一步加剧, 使碳原子取代SiO4中的3~4个氧原子而生成SiC3O和β-SiC。结合SEM照片发现, 处理温度低于1200 ℃时, 气凝胶微观形貌变化不大, 依然保持良好的颗粒堆积结构; 而1400 ℃处理后气凝胶的颗粒略有减小, 颗粒间出现了大量丝状SiC纳米线[30], 与前文分析结果对应。
图5
图5
碳化前后样品V/VM-3的照片
Fig. 5
Pictures of sample V/VM-3 before and after carbonization
2.4 无机Si-O-C气凝胶的抗氧化性能
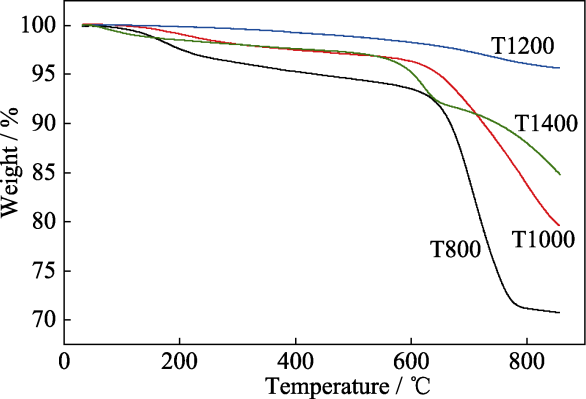
将上述经过800、1000、1200和1400 ℃处理后具有Si-O-C结构和β-SiC纳米线的无机气凝胶分别命名为T800、T1000、T1200和T1400, 通过测试空气氛围下的TG曲线探究了几种不同无机气凝胶的耐高温氧化性能, 结果如图6所示。样品T800是无机SiO2和游离碳的混合体, 630 ℃以下的缓慢失重是由游离碳吸附的水及氢氧化合物挥发引起的, 在630 ℃左右发生游离碳氧化而导致急剧失重, 到800 ℃左右游离碳烧蚀殆尽其热重趋于稳定。而样品T1000由于碳热还原反应消耗掉部分游离碳, 使630 ℃上下的失重都减少。样品T1200中几乎所有游离碳都通过碳热还原反应生成了稳定的SiO4、SiCO3和SiC2O2结构, 所以其热失重最小, 仅为4%。样品T1400由于含吸水性较强的β-SiC, 所以升温初期失重较明显, 升至560 ℃出现的快速失重主要是由于SiC3O氧化引起的; 升至640 ℃左右样品失重开始减缓, 这可能是由于β-SiC纳米线的比表面积高, 在有氧环境中的氧化反应性大大增强, 在较低温度下就被氧化为无机SiO2而引起增重, 使样品整体失重减缓[31]。
图6
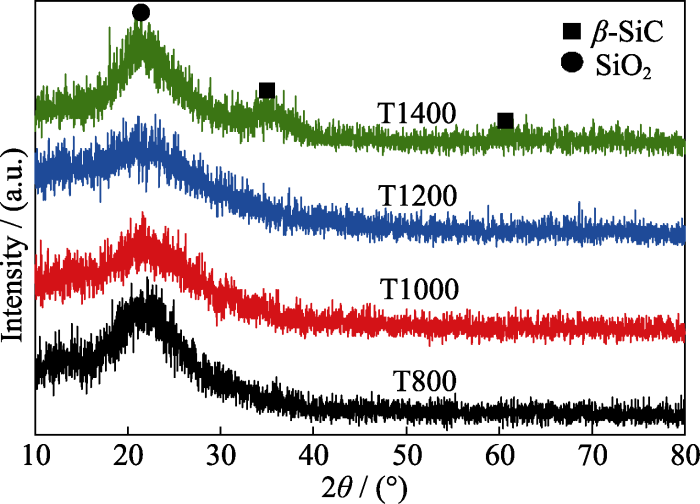
图7为T800、T1000、T1200和T1400四个样品经800 ℃氧化处理后的XRD谱图。样品T800由于游离碳的氧化挥发, 无机SiO2纯度提高使其XRD特征峰更明显; 样品T1000和T1200在氧化处理后也出现微弱的SiO2特征峰, 这是由于样品Si-O-C结构中含有的极少量SiC3O氧化生成SiO2导致的; T1400在氧化处理后, β-SiC峰已基本消失, 并出现明显SiO2特征峰, 说明β-SiC及SiC3O在热处理过程中被氧化成无机SiO2, 这与热重结果对应。另外通过800 ℃氧化处理后T1400样品的SEM照片也可以发现, β-SiC纳米线在氧化热处理后基本消失, 只残余原始的颗粒堆积结构。
图7
图7
无机Si-O-C气凝胶在800 ℃氧化处理后的XRD谱图
Fig. 7
XRD patterns of inorganic Si-O-C aerogels after oxidized at 800 ℃
图8
图8
样品T1400经800 ℃氧化处理后的SEM照片
Fig. 8
SEM image of sample T1400 after oxidized at 800 ℃
3 结论
基于有机-无机杂化改善气凝胶力学性能的方法, 以乙烯基三甲氧基硅烷(VTMS)和乙烯基甲基二甲氧基硅烷(VMDMS)为硅源, 制备了柔性良好的有机硅气凝胶, 研究了其微观孔结构的调控方法和高温无机化转变过程, 结果表明:
1)前驱体中三官能度的VTMS比例增加有助于提高气凝胶分子网络中Si-O-Si交联程度, 使气凝胶颗粒减小且堆积更紧密, 其压缩回弹性能也随之降低。
2)在空气氛围下气凝胶从210 ℃开始发生乙烯基氧化, 约400 ℃发生甲基的氧化, 随温度进一步升高主链Si-O-Si断裂、重排, 到800 ℃气凝胶基本被氧化为无机SiO2, 残重约69%。
3)在N2氛围下气凝胶从480 ℃开始发生有机侧链裂解; 到800 ℃裂解基本完成, 残余SiO2和游离碳达92%; 1000~1200 ℃进一步处理后SiO2和游离碳经碳热还原反应生成SiO4、SiCO3和SiC2O2等无定形的Si-O-C结构; 1400 ℃碳热还原反应进一步发生, 生成SiC3O结构和少量β-SiC纳米线。
4)经1200 ℃碳化处理后的气凝胶可形成具有良好耐高温氧化性能的Si-O-C结构, 可为制备耐高温氧化无机气凝胶提供参考。
参考文献
Highly compressible, anisotropic aerogel with aligned cellulose nanofibers
Aerogels can be used in a broad range of applications such as bioscaffolds, energy storage devices, sensors, pollutant treatment, and thermal insulating materials due to their excellent properties including large surface area, low density, low thermal conductivity, and high porosity. Here we report a facile and effective top-down approach to fabricate an anisotropic wood aerogel directly from natural wood by a simple chemical treatment. The wood aerogel has a layered structure with anisotropic structural properties due to the destruction of cell walls by the removal of lignin and hemicellulose. The layered structure results in the anisotropic wood aerogel having good mechanical compressibility and fragility resistance, demonstrated by a high reversible compression of 60% and stress retention of ∼90% after 10 000 compression cycles. Moreover, the anisotropic structure of the wood aerogel with curved layers stacking layer-by-layer and aligned cellulose nanofibers inside each individual layer enables the wood aerogel to have an anisotropic thermal conductivity with an anisotropy factor of ∼4.3. An extremely low thermal conductivity of 0.028 W/m·K perpendicular to the cellulose alignment direction and a thermal conductivity of 0.12 W/m·K along the cellulose alignment direction can be achieved. The thermal conductivity is not only much lower than that of the natural wood material (by ∼3.6 times) but also lower than most of the commercial thermal insulation materials. The top-down approach is low-cost, scalable, simple, yet effective, representing a promising direction for the fabrication of high-quality aerogel materials.
Bioinspired synthesis of monolithic and layered aerogels
Carbon nitride aerogels for the photoredox conversion of water
Emerging hierarchical aerogels: self-assembly of metal and semiconductor nanocrystals
Facile synthesis of three-dimensional Pt-TiO2 nano-networks: a highly active catalyst for the hydrolytic dehydrogenation of ammonia-borane
Catalytically doped semiconductors for chemical gas sensing: aerogel-like aluminum- containing zinc oxide materials prepared in the gas phase
Aerogels-airy materials: chemistry, structure, and properties
Adsorption and desorption of helium in aerogels
Aerogel as cherenkov radiator for rich detectors
A special material or new state of matter: a review and reconsideration of the aerogel
An overview on silica aerogels synthesized by siloxane co-precursors
Due to their unique features, such as high specific surface area, high porosity, low density, low thermal conductivity, and high transmittance, aerogels can be widely applied in the fields of thermal insulation, sound insulation and optics. However, aerogels usually tend to be destructive collapse, due to their porous structures constituted by slightly brittle skeletons, which is a negative factor to restrict their applications. According to the number and variety of non-hydrolytic groups in siloxane precursors, an overview of the literatures is presented on silica aerogels synthesized by three kinds of siloxane co-precursors, i.e. integral hydrolytic co-precursors, integral/partial and partial hydrolytic co-precursors. The characteristics of porous structures and properties in mechanics, thermal insulation, optics, and hydrophobicity are analyzed. It is an effective method by choosing appropriate precursors to realize the designs and improve mechanical behaviors of aerogels in respect of structures and properties.
Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels
New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties
Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor
Transparent, superflexible doubly cross-linked polyvinylpolymethylsiloxane aerogel superinsulators via ambient pressure drying
Preparation, mechanical properties and thermal properties of elastic aerogels
Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions
A superamphiphobic macroporous silicone monolith with marshmallow- like flexibility
Study of 29Si MAS NMR spectroscopy and electro-optic property based on polyimide/SiO2
Transparent ethylene-bridged polymethylsiloxane aerogels and xerogels with improved bending flexibility
Transparent, monolithic aerogels with nanosized colloidal skeletons have been obtained from a single precursor of 1,2-bis(methyldiethoxysilyl)ethane (BMDEE) by adopting a liquid surfactant and a two-step process involving strong-acid, followed by strong-base, sol-gel reactions. This precursor BMDEE forms the ethylene-bridged polymethylsiloxane (EBPMS, O(CH)Si-CHCH-Si(CH)O) network, in which each silicon has one methyl, two bridging oxygens, and one bridging ethylene, exhibiting an analogous structure to that of the previously reported polymethylsilsesquioxane (PMSQ, CHSiO) aerogels having one methyl and three bridging oxygen atoms. Obtained aerogels consist of fine colloidal skeletons and show high visible-light transparency and a flexible deformation behavior against compression without collapse. Similar to the PMSQ aerogels, a careful tuning of synthetic conditions can produce low-density (0.19 g cm) and highly transparent (76% at 550 nm, corresponding to 10 mm thick samples) xerogels via ambient pressure drying by solvent evaporation due to their high strength and resilience against compression. Moreover, EBPMS aerogels exhibit higher bending strength and bending strain at break against the three-point bending mode compared to PMSQ aerogels. This improved bendability is presumably derived from the introduced ethylene-bridging parts, suggesting the potential for realizing transparent and bendable aerogels in such polysiloxane materials with organic linking units.
Effect of organic-inorganic crosslinking degree on the mechanical and thermal properties of aerogels
In all kinds of aerogels, silicon-based aerogel possesses the most comprehensive mechanism of Sol-Gel process and maturest synthesis process. In this work, different types of silicon-based aerogels were prepared via different silicon precursors including tetraethoxysilane (TEOS), methyltrimethoxysilane (MTMS), vinylmethyldimethoxysilane (VMDMS) and the mixed precursor of MTMS and dimethyldimethoxysilane (DMDMS). All samples display high specific surface area and porous microstructure. Effect of the precursor structure on the mechanical and thermal properties of final samples was deeply investigated. The results show that elastic property of silicon-based aerogels depends greatly on the crosslinking degree of their skeleton. The lower the crosslinking degree of the sample is, the better the elastic property is. Furthermore, the elastic property can be further improved by introducing hydrocarbon chains into the skeleton. Thermal conductivity of the obtained aerogels is between 0.032 and 0.041 W/(m·K) at room temperature. Their weight loss increases with the increase of the organic component in the skeleton. Superior mechanical and thermal properties enable silicon-based aerogels to be promising candidates for thermal insulation and energy storage.
Superelastic triple-network polyorganosiloxane-based aerogels as transparent thermal superinsulators and efficient separators
Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application
Resilient, fire-retardant and mechanically strong polyimide-polyvinylpolymethylsiloxane composite aerogel prepared via stepwise chemical liquid deposition
Pyrolysis of phenolic resin by TG-MS and FTIR analysis
Characteristics of nanoporous silica aerogel under high temperature from 950 ℃ to 1200 ℃
Computer simulation of the aggregation and sintering restructuring of fractal-like clusters containing limited numbers of primary particles
Synthesis of nanometre silicon carbide whiskers from binary carbonaceous silica aerogels